Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents
- PMID: 22244599
- PMCID: PMC3294274
- DOI: 10.1016/j.jaci.2011.12.962
Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents
Abstract
Background: Asthma severity is reflected in many aspects of the disease, including impairment and future risks, particularly for exacerbations. According to the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, however, to assess more comprehensively the severity of asthma the level of current treatment needed to maintain a level of control should be included.
Objective: Development and validation of a new instrument, the Composite Asthma Severity Index (CASI), which can quantify disease severity by taking into account impairment, risk, and the amount of medication needed to maintain control. At present, there is no instrument available to measure and assess the multidimensional nature of asthma.
Methods: Twenty-six established asthma investigators, who are part of the National Institutes of Health-supported Inner City Asthma Consortium, participated in a modified Delphi consensus process to identify and weight the dimensions of asthma. Factor analysis was performed to identify independent domains of asthma by using the Asthma Control Evaluation trial. CASI was validated by using the Inner City Anti-IgE Therapy for Asthma trial.
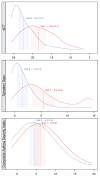
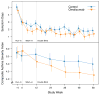
Results: CASI scores include 5 domains: day symptoms and albuterol use, night symptoms and albuterol use, controller treatment, lung function measures, and exacerbations. At Asthma Control Evaluation trial enrollment, CASI ranged from 0 to 17, with a mean of 6.2. CASI was stable, with minimal change in variance after 1 year of treatment. In external validation, CASI detected a 32% larger improvement than did symptoms alone.
Conclusion: CASI retained its discriminatory ability even with low levels of symptoms reported after months of guidelines-directed care. Thus, CASI has the ability to determine the level of asthma severity and provide a composite clinical characterization of asthma.
Published by Mosby, Inc.
Figures


References
-
- National Heart, Lung, and Blood Institute. [Accessed December 1, 2011];Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma - Full Report 2007. 2007 August 28; Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
-
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-JAI-25482/PHS HHS/United States
- N01 AI025496/AI/NIAID NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- RR00052/RR/NCRR NIH HHS/United States
- M01RR00071/RR/NCRR NIH HHS/United States
- 1UL1RR024156/RR/NCRR NIH HHS/United States
- 5UL1RR024992-02/RR/NCRR NIH HHS/United States
- M01RR00533/RR/NCRR NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- 1UL1RR025771/RR/NCRR NIH HHS/United States
- 5M01RR020359-04/RR/NCRR NIH HHS/United States
- N01-AI-25496/AI/NIAID NIH HHS/United States
- M01 RR020359/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

