The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition
- PMID: 22244757
- PMCID: PMC3378326
- DOI: 10.1016/j.str.2011.10.022
The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition
Abstract
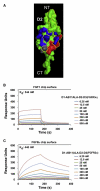
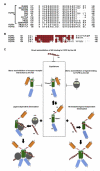
Uncontrolled fibroblast growth factor (FGF) signaling can lead to human malignancies necessitating multiple layers of self-regulatory control mechanisms. Fibroblast growth factor receptor (FGFR) autoinhibition mediated by the alternatively spliced immunoglobulin (Ig) domain 1 (D1) and the acid box (AB)-containing linker between D1 and Ig domain 2 (D2) serves as the first line of defense to minimize inadvertent FGF signaling. In this report, nuclear magnetic resonance and surface plasmon resonance spectroscopy are used to demonstrate that the AB subregion of FGFR electrostatically engages the heparan sulfate (HS)-binding site on the D2 domain in cis to directly suppress HS-binding affinity of FGFR. Furthermore, the cis electrostatic interaction sterically autoinhibits ligand-binding affinity of FGFR because of the close proximity of HS-binding and primary ligand-binding sites on the D2 domain. These data, together with the strong amino acid sequence conservation of the AB subregion among FGFR orthologs, highlight the universal role of the AB subregion in FGFR autoinhibition.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Heparan sulfate fibroblast growth factor receptor complex: structure-function relationships.Mol Reprod Dev. 1994 Sep;39(1):69-81; discusison 81-2. doi: 10.1002/mrd.1080390112. Mol Reprod Dev. 1994. PMID: 7999363 Review.
-
Compositional analysis of heparin/heparan sulfate interacting with fibroblast growth factor.fibroblast growth factor receptor complexes.Biochemistry. 2009 Sep 8;48(35):8379-86. doi: 10.1021/bi9006379. Biochemistry. 2009. PMID: 19591432 Free PMC article.
-
Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity.Proc Natl Acad Sci U S A. 2004 Jan 27;101(4):935-40. doi: 10.1073/pnas.0307287101. Epub 2004 Jan 19. Proc Natl Acad Sci U S A. 2004. PMID: 14732692 Free PMC article.
-
Common and specific determinants for fibroblast growth factors in the ectodomain of the receptor kinase complex.Biochemistry. 1999 Jan 5;38(1):160-71. doi: 10.1021/bi981758m. Biochemistry. 1999. PMID: 9890894
-
A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs.Curr Opin Struct Biol. 2005 Oct;15(5):506-16. doi: 10.1016/j.sbi.2005.09.002. Curr Opin Struct Biol. 2005. PMID: 16154740 Review.
Cited by
-
Quantitative and qualitative differences in the activation of a fibroblast growth factor receptor by different FGF ligands.Cytokine Growth Factor Rev. 2024 Aug;78:77-84. doi: 10.1016/j.cytogfr.2024.07.002. Epub 2024 Jul 9. Cytokine Growth Factor Rev. 2024. PMID: 39043538 Free PMC article. Review.
-
FGF/FGFR Signaling in Hepatocellular Carcinoma: From Carcinogenesis to Recent Therapeutic Intervention.Cancers (Basel). 2021 Mar 17;13(6):1360. doi: 10.3390/cancers13061360. Cancers (Basel). 2021. PMID: 33802841 Free PMC article. Review.
-
Cryo-EM structure of human type-3 inositol triphosphate receptor reveals the presence of a self-binding peptide that acts as an antagonist.J Biol Chem. 2020 Feb 7;295(6):1743-1753. doi: 10.1074/jbc.RA119.011570. Epub 2020 Jan 8. J Biol Chem. 2020. PMID: 31915246 Free PMC article.
-
Somatic rearrangements causing oncogenic ectodomain deletions of FGFR1 in squamous cell lung cancer.J Clin Invest. 2023 Nov 1;133(21):e170217. doi: 10.1172/JCI170217. J Clin Invest. 2023. PMID: 37606995 Free PMC article.
-
FGF-based drug discovery: advances and challenges.Nat Rev Drug Discov. 2025 May;24(5):335-357. doi: 10.1038/s41573-024-01125-w. Epub 2025 Jan 28. Nat Rev Drug Discov. 2025. PMID: 39875570 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

