The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug
- PMID: 22244854
- PMCID: PMC3350832
- DOI: 10.1016/j.jmb.2011.12.060
The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug
Abstract
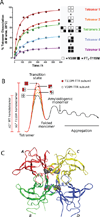
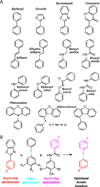
Transthyretin (TTR) is one of the many proteins that are known to misfold and aggregate (i.e., undergo amyloidogenesis) in vivo. The process of TTR amyloidogenesis causes nervous system and/or heart pathology. While several of these maladies are associated with mutations that destabilize the native TTR quaternary and/or tertiary structure, wild-type TTR amyloidogenesis also leads to the degeneration of postmitotic tissue. Over the past 20 years, much has been learned about the factors that influence the propensity of TTR to aggregate. This biophysical information led to the development of a therapeutic strategy, termed "kinetic stabilization," to prevent TTR amyloidogenesis. This strategy afforded the drug tafamidis which was recently approved by the European Medicines Agency for the treatment of TTR familial amyloid polyneuropathy, the most common familial TTR amyloid disease. Tafamidis is the first and currently the only medication approved to treat TTR familial amyloid polyneuropathy. Here we review the biophysical basis for the kinetic stabilization strategy and the structure-based drug design effort that led to this first-in-class pharmacologic agent.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




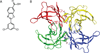

Similar articles
-
Semi-quantitative models for identifying potent and selective transthyretin amyloidogenesis inhibitors.Bioorg Med Chem Lett. 2017 Aug 1;27(15):3441-3449. doi: 10.1016/j.bmcl.2017.05.080. Epub 2017 May 26. Bioorg Med Chem Lett. 2017. PMID: 28625364 Free PMC article.
-
AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin.Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):9992-7. doi: 10.1073/pnas.1300761110. Epub 2013 May 28. Proc Natl Acad Sci U S A. 2013. PMID: 23716704 Free PMC article.
-
Specific disruption of transthyretin(105-115) fibrilization using "stabilizing" inhibitors of transthyretin amyloidogenesis.Biochemistry. 2012 Apr 24;51(16):3523-30. doi: 10.1021/bi3002727. Epub 2012 Apr 12. Biochemistry. 2012. PMID: 22482799
-
Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses.Acc Chem Res. 2005 Dec;38(12):911-21. doi: 10.1021/ar020073i. Acc Chem Res. 2005. PMID: 16359163 Review.
-
Mechanism of Action and Clinical Application of Tafamidis in Hereditary Transthyretin Amyloidosis.Neurol Ther. 2016 Jun;5(1):1-25. doi: 10.1007/s40120-016-0040-x. Epub 2016 Feb 19. Neurol Ther. 2016. PMID: 26894299 Free PMC article. Review.
Cited by
-
Expanding proteostasis by membrane trafficking networks.Cold Spring Harb Perspect Biol. 2013 Jul 1;5(7):a013383. doi: 10.1101/cshperspect.a013383. Cold Spring Harb Perspect Biol. 2013. PMID: 23426524 Free PMC article. Review.
-
Brain Permeable Tafamidis Amide Analogs for Stabilizing TTR and Reducing APP Cleavage.ACS Med Chem Lett. 2020 Mar 12;11(10):1973-1979. doi: 10.1021/acsmedchemlett.9b00688. eCollection 2020 Oct 8. ACS Med Chem Lett. 2020. PMID: 33062181 Free PMC article.
-
Familial amyloidotic polyneuropathy: current and emerging treatment options for transthyretin-mediated amyloidosis.Appl Clin Genet. 2012 Jun 18;5:37-41. doi: 10.2147/TACG.S19903. Print 2012. Appl Clin Genet. 2012. PMID: 23776379 Free PMC article.
-
Inhibition of human transthyretin aggregation by non-steroidal anti-inflammatory compounds: a structural and thermodynamic analysis.Int J Mol Sci. 2013 Mar 6;14(3):5284-311. doi: 10.3390/ijms14035284. Int J Mol Sci. 2013. PMID: 23466880 Free PMC article.
-
Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation.Nat Commun. 2015 Jun 16;6:7314. doi: 10.1038/ncomms8314. Nat Commun. 2015. PMID: 26076669 Free PMC article.
References
-
- Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J. Mol. Biol. 1978;121:339–356. - PubMed
-
- Hornberg A, Eneqvist T, Olofsson A, Lundgren E, Sauer-Eriksson AE. A comparative analysis of 23 structures of the amyloidogenic protein transthyretin. J. Mol. Biol. 2000;302:649–669. - PubMed
-
- Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

