The vestibular system: multimodal integration and encoding of self-motion for motor control
- PMID: 22245372
- PMCID: PMC4000483
- DOI: 10.1016/j.tins.2011.12.001
The vestibular system: multimodal integration and encoding of self-motion for motor control
Abstract
Understanding how sensory pathways transmit information under natural conditions remains a major goal in neuroscience. The vestibular system plays a vital role in everyday life, contributing to a wide range of functions from reflexes to the highest levels of voluntary behavior. Recent experiments establishing that vestibular (self-motion) processing is inherently multimodal also provide insight into a set of interrelated questions. What neural code is used to represent sensory information in vestibular pathways? How do the interactions between the organism and the environment shape encoding? How is self-motion information processing adjusted to meet the needs of specific tasks? This review highlights progress that has recently been made towards understanding how the brain encodes and processes self-motion to ensure accurate motor control.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
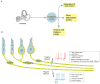


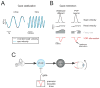
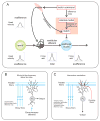
References
-
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annual review of neuroscience. 2008;31:125–150. - PubMed
-
- Eatock RA, Songer JE. Vestibular Hair Cells and Afferents: Two Channels for Head Motion Signals. Annual review of neuroscience. 2011;34:501–534. - PubMed
-
- Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. Journal of computational neuroscience. 2001;11:217–239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

