The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy
- PMID: 22245557
- PMCID: PMC3286596
- DOI: 10.1016/j.biomaterials.2011.12.052
The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy
Abstract
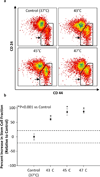
Breast tumors contain a small population of tumor initiating stem-like cells, termed breast cancer stem cells (BCSCs). These cells, which are refractory to chemotherapy and radiotherapy, are thought to persist following treatment and drive tumor recurrence. We examined whether BCSCs are similarly resistant to hyperthermic therapy, and whether nanoparticles could be used to overcome this resistance. Using a model of triple-negative breast cancer stem cells, we show that BCSCs are markedly resistant to traditional hyperthermia and become enriched in the surviving cell population following treatment. In contrast, BCSCs are sensitive to nanotube-mediated thermal treatment and lose their long-term proliferative capacity after nanotube-mediated thermal therapy. Moreover, use of this therapy in vivo promotes complete tumor regression and long-term survival of mice bearing cancer stem cell-driven breast tumors. Mechanistically, nanotube thermal therapy promotes rapid membrane permeabilization and necrosis of BCSCs. These data suggest that nanotube-mediated thermal treatment can simultaneously eliminate both the differentiated cells that constitute the bulk of a tumor and the BCSCs that drive tumor growth and recurrence.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





References
-
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. - PubMed
-
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

