Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2
- PMID: 22245792
- PMCID: PMC3335957
- DOI: 10.1016/j.hrthm.2012.01.010
Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2
Abstract
Background: Ranolazine (Ran) is known to inhibit multiple targets, including the late Na(+)current, the rapid delayed rectifying K(+)current, the L-type Ca(2+)current, and fatty acid metabolism. Functionally, Ran suppresses early afterdepolarization (EADs) and torsades de pointes (TdP) in drug-induced long QT type 2 (LQT2) presumably by decreasing intracellular [Na(+)](i) and Ca(2+)overload. However, simulations of EADs in LQT2 failed to predict their suppression by Ran.
Objective: To elucidate the mechanism(s) whereby Ran alters cardiac action potentials (APs) and cytosolic Ca(2+)transients and suppresses EADs and TdP in LQT2.
Methods: The known effects of Ran were included in simulations (Shannon and Mahajan models) of rabbit ventricular APs and Ca(2+)transients in control and LQT2 models and compared with experimental optical mapping data from Langendorff rabbit hearts treated with E4031 (0.5 μM) to block the rapid delayed rectifying K(+)current. Direct effects of Ran on cardiac ryanodine receptors (RyR2) were investigated in single channels and changes in Ca(2+)-dependent high-affinity ryanodine binding.
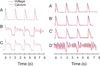
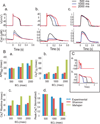
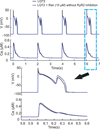
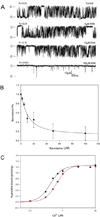
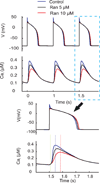
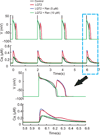
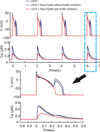
Results: Ran (10 μM) alone prolonged action potential durations (206 ± 4.6 to 240 ± 7.8 ms; P <0.05); E4031 prolonged action potential durations (204 ± 6 to 546 ± 35 ms; P <0.05) and elicited EADs and TdP that were suppressed by Ran (10 μM; n = 7 of 7 hearts). Simulations (Shannon but not Mahajan model) closely reproduced experimental data except for EAD suppression by Ran. Ran reduced open probability (P(o)) of RyR2 (half maximal inhibitory concentration = 10 ± 3 μM; n = 7) in bilayers and shifted half maximal effective concentration for Ca(2+)-dependent ryanodine binding from 0.42 ± 0.02 to 0.64 ± 0.02 μM with 30 μM Ran.
Conclusions: Ran reduces P(o) of RyR2, desensitizes Ca(2+)-dependent RyR2 activation, and inhibits Ca(i) oscillations, which represents a novel mechanism for its suppression of EADs and TdP.
Copyright © 2012 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts to disclose
Figures
References
-
- Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. Journal of Molecular and Cellular Cardiology. 1996 Feb;28:341–350. - PubMed
-
- McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996 Jan 1;93:135–142. - PubMed
-
- Sabbah HN, Chandler MP, Mishima T, et al. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. Journal of Cardiac Failure. 2002 Dec;8:416–422. - PubMed
-
- Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. Journal of Pharmacology and Experimental Therapeutics. 2004 Aug;310:599–605. - PubMed
-
- Antoons G, Oros A, Beekman JD, et al. Late na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. Journal of the American College of Cardiology. 2010 Feb 23;55:801–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

