Understanding silibinin's modes of action against HCV using viral kinetic modeling
- PMID: 22245888
- PMCID: PMC3328661
- DOI: 10.1016/j.jhep.2011.12.012
Understanding silibinin's modes of action against HCV using viral kinetic modeling
Abstract
Background & aims: Legalon® SIL (SIL) is a chemically hydrophilized version of silibinin that has exhibited high antiviral effectiveness against hepatitis C virus (HCV). Its main mode of action (MOA) remains unclear, with contradicting in vitro studies supporting either suppression of entry and cell-to-cell spread or suppression of viral RNA synthesis as the main MOA. We sought to provide new insights into SIL's MOA in HCV genotype-1/4 patients receiving intravenous SIL monotherapy for 7 days via mathematical modeling.
Methods: Changes in HCV RNA in 25 patients receiving 10, 15, or 20mg/kg/day of SIL were analyzed and modeled using viral kinetic methods.
Results: In 15 patients, the virus declined in a biphasic manner, in which a sharp drop between days 0 and 2 was followed by a slower second phase of decline. In 10 patients, the initial decline was weaker and the virus declined in a single phase over the 7-day period. The blocking production effectiveness, ε, was dose-dependent with mean ε=0.49 and 0.89 in the 10 or 15 and 20mg/kg/day dosing groups, respectively (p=0.02). The effectiveness of blocking viral infection, η, was estimated as 0.60 with no significant differences across dosing groups. For all patients, the mean rate of viral load decline measured between days 2 and 7 was high (0.3 log(10)IU/ml/day), i.e., 4-fold higher than typically observed during the 2nd phase of (pegylated)-interferon-α±ribavirin treatment.
Conclusions: Modeling HCV kinetics in vivo suggests that SIL may block both viral infection and viral production/release with its main dose-dependent effect being blocking viral production/release.
Copyright © 2012 European Association for the Study of the Liver. All rights reserved.
Figures
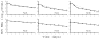
References
-
- World Health Organization. Hepatitis C Fact sheet No 164. Revised June 2011. http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
-
- Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–657. - PubMed
-
- Awad T, Thorlund K, Hauser G, Mabrouk M, Gluud C. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: a systematic review of randomized trials. Hepatology. 2010;51:1176–1184. - PubMed
-
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–1838. - PubMed
-
- McHutchison J, Manns M, Muir A, Terrault N, Jacobson I, Afdhal N, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI078881/AI/NIAID NIH HHS/United States
- AI028433/AI/NIAID NIH HHS/United States
- R56 AI065256/AI/NIAID NIH HHS/United States
- R37 AI028433/AI/NIAID NIH HHS/United States
- R01 RR006555/RR/NCRR NIH HHS/United States
- P20 RR018754/RR/NCRR NIH HHS/United States
- AI065256/AI/NIAID NIH HHS/United States
- R01 AI078881/AI/NIAID NIH HHS/United States
- R56 AI078881/AI/NIAID NIH HHS/United States
- P20-RR018754/RR/NCRR NIH HHS/United States
- R01 AI065256/AI/NIAID NIH HHS/United States
- R01 OD011095/OD/NIH HHS/United States
- RR006555/RR/NCRR NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

