The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation
- PMID: 22245967
- PMCID: PMC3272117
- DOI: 10.1038/nsmb.2207
The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation
Abstract
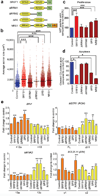
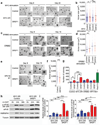
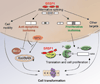
The splicing-factor oncoprotein SRSF1 (also known as SF2/ASF or ASF/SF2) is upregulated in breast cancers. We investigated the ability of SRSF1 to transform human and mouse mammary epithelial cells in vivo and in vitro. SRSF1-overexpressing COMMA-1D cells formed tumors, following orthotopic transplantation to reconstitute the mammary gland. In three-dimensional (3D) culture, SRSF1-overexpressing MCF-10A cells formed larger acini than control cells, reflecting increased proliferation and delayed apoptosis during acinar morphogenesis. These effects required the first RNA-recognition motif and nuclear functions of SRSF1. SRSF1 overexpression promoted alternative splicing of BIM (also known as BCL2L11) and BIN1 to produce isoforms that lack pro-apoptotic functions and contribute to the phenotype. Finally, SRSF1 cooperated specifically with MYC to transform mammary epithelial cells, in part by potentiating eIF4E activation, and these cooperating oncogenes are significantly coexpressed in human breast tumors. Thus, SRSF1 can promote breast cancer, and SRSF1 itself or its downstream effectors may be valuable targets for the development of therapeutics.
Figures




References
-
- Cartegni L, Chew SL, Krainer AR. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. - PubMed
-
- Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–375. - PubMed
-
- Caceres JF, Stamm S, Helfman DM, Krainer AR. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. - PubMed
-
- Srebrow A, Kornblihtt AR. The connection between splicing and cancer. J Cell Sci. 2006;119:2635–2641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

