Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury
- PMID: 22246174
- PMCID: PMC3326286
- DOI: 10.1164/rccm.201108-1533OC
Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury
Expression of concern in
-
Expression of Concern: Disruption of Platelet-derived Chemokine Heteromers Prevents Neutrophil Extravasation in Acute Lung Injury.Am J Respir Crit Care Med. 2020 Oct 15;202(8):1199. doi: 10.1164/rccm.v202eoc1. Am J Respir Crit Care Med. 2020. PMID: 33054328 Free PMC article. No abstract available.
Abstract
Rationale: Acute lung injury (ALI) causes high mortality, but its molecular mechanisms and therapeutic options remain ill-defined. Gram-negative bacterial infections are the main cause of ALI, leading to lung neutrophil infiltration, permeability increases, deterioration of gas exchange, and lung damage. Platelets are activated during ALI, but insights into their mechanistic contribution to neutrophil accumulation in the lung are elusive.
Objectives: To determine mechanisms of platelet-mediated neutrophil recruitment in ALI.
Methods: Interference with platelet-neutrophil interactions using antagonists to P-selectin and glycoprotein IIb/IIIa or a small peptide antagonist disrupting platelet chemokine heteromer formation in mouse models of ALI.
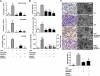
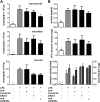
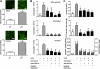
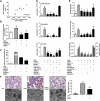
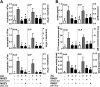
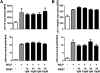
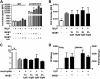
Measurements and main results: In a murine model of LPS-induced ALI, we uncover important roles for neutrophils and platelets in permeability changes and subsequent lung damage. Furthermore, platelet depletion abrogated lung neutrophil infiltration, suggesting a sequential participation of platelets and neutrophils. Whereas antagonists to P-selectin and glycoprotein IIb/IIIa had no effects on LPS-mediated ALI, antibodies to the platelet-derived chemokines CCL5 and CXCL4 strongly diminished neutrophil eflux and permeability changes. The two chemokines were found to form heteromers in human and murine ALI samples, positively correlating with leukocyte influx into the lung. Disruption of CCL5-CXCL4 heteromers in LPS-, acid-, and sepsis-induced ALI abolished lung edema, neutrophil infiltration, and tissue damage, thereby revealing a causal contribution.
Conclusions: Taken together, our data identify a novel function of platelet-derived chemokine heteromers during ALI and demonstrate means for therapeutic interference.
Figures
References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 - PubMed
-
- Soehnlein O, Oehmcke S, Ma X, Rothfuchs AG, Frithiof R, van Rooijen N, Mörgelin M, Herwald H, Lindbom L. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J 2008;32:405–412 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

