Axonal transcription factors signal retrogradely in lesioned peripheral nerve
- PMID: 22246183
- PMCID: PMC3321171
- DOI: 10.1038/emboj.2011.494
Axonal transcription factors signal retrogradely in lesioned peripheral nerve
Abstract
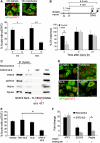
Retrograde axonal injury signalling stimulates cell body responses in lesioned peripheral neurons. The involvement of importins in retrograde transport suggests that transcription factors (TFs) might be directly involved in axonal injury signalling. Here, we show that multiple TFs are found in axons and associate with dynein in axoplasm from injured nerve. Biochemical and functional validation for one TF family establishes that axonal STAT3 is locally translated and activated upon injury, and is transported retrogradely with dynein and importin α5 to modulate survival of peripheral sensory neurons after injury. Hence, retrograde transport of TFs from axonal lesion sites provides a direct link between axon and nucleus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
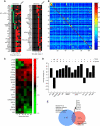
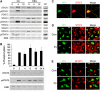
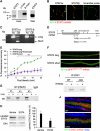
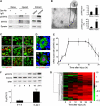

Comment in
-
Ready, STAT, go: transcription factors on the move.EMBO J. 2012 Mar 21;31(6):1331-3. doi: 10.1038/emboj.2012.34. Epub 2012 Feb 14. EMBO J. 2012. PMID: 22333913 Free PMC article.
References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502 - PubMed
-
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM (2001) Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18: 270–282 - PubMed
-
- Andreassi C, Zimmermann C, Mitter R, Fusco S, De Vita S, Saiardi A, Riccio A (2010) An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat Neurosci 13: 291–301 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

