Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation
- PMID: 22246186
- PMCID: PMC3297999
- DOI: 10.1038/emboj.2011.492
Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation
Abstract
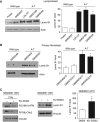
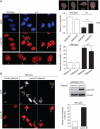
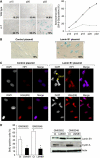
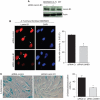
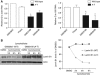
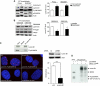
We report crosstalk between three senescence-inducing conditions, DNA damage response (DDR) defects, oxidative stress (OS) and nuclear shape alterations. The recessive autosomal genetic disorder Ataxia telangiectasia (A-T) is associated with DDR defects, endogenous OS and premature ageing. Here, we find frequent nuclear shape alterations in A-T cells, as well as accumulation of the key nuclear architecture component lamin B1. Lamin B1 overexpression is sufficient to induce nuclear shape alterations and senescence in wild-type cells, and normalizing lamin B1 levels in A-T cells reciprocally reduces both nuclear shape alterations and senescence. We further show that OS increases lamin B1 levels through p38 Mitogen Activated Protein kinase activation. Lamin B1 accumulation and nuclear shape alterations also occur during stress-induced senescence and oncogene-induced senescence (OIS), two canonical senescence situations. These data reveal lamin B1 as a general molecular mediator that controls OS-induced senescence, independent of established Ataxia Telangiectasia Mutated (ATM) roles in OIS.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
B-type lamins and their elusive roles in metazoan cell proliferation and senescence.EMBO J. 2012 Mar 7;31(5):1058-9. doi: 10.1038/emboj.2012.39. Epub 2012 Feb 17. EMBO J. 2012. PMID: 22343942 Free PMC article.
References
-
- Bartek J, Bartkova J, Lukas J (2007) DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 26: 7773–7779 - PubMed
-
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J et al. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444: 633–637 - PubMed
-
- Barzilai A, Biton S, Shiloh Y (2008) The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair (Amst) 7: 1010–1027 - PubMed
-
- Barzilai A, Rotman G, Shiloh Y (2002) ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 1: 3–25 - PubMed
-
- Biton S, Barzilai A, Shiloh Y (2008) The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst) 7: 1028–1038 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

