Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells
- PMID: 22246562
- PMCID: PMC3583532
- DOI: 10.3892/or.2012.1630
Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells
Abstract
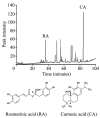
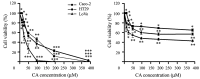
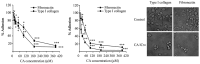
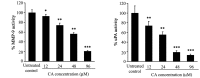
Colorectal cancer (CRC) is the third most common malignant neoplasm worldwide. The objective of this study was to examine whether carnosic acid (CA), the main antioxidant compound of Rosmarinus officinalis L., would inhibit the cell viability of three CRC cell lines: Caco-2, HT29 and LoVo in a dose-dependent manner, with IC₅₀ values in the range of 24-96 µM. CA induced cell death by apoptosis in Caco-2 line after 24 h of treatment and inhibited cell adhesion and migration, possibly by reducing the activity of secreted proteases such as urokinase plasminogen activator (uPA) and metalloproteinases (MMPs). These effects may be associated through a mechanism involving the inhibition of the COX-2 pathway, because we have determined that CA downregulates the expression of COX-2 in Caco-2 cells at both the mRNA and protein levels. Therefore, CA modulates different targets involved in the development of CRC. These findings indicate that carnosic acid may have anticancer activity and may be useful as a novel chemotherapeutic agent.
Figures



Comment in
-
Carnosic acid and its inhibitory effect on tumor growth in systemic malignancies.Oral Dis. 2013 May;19(4):427. doi: 10.1111/odi.12055. Epub 2012 Dec 28. Oral Dis. 2013. PMID: 23279589 No abstract available.
References
-
- Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271–1279. - PubMed
-
- Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. - PubMed
-
- Kundu JK, Surh YJ. Epigallocatechin gallate inhibits phorbol ester-induced activation of NF-κB and CREB in mouse skin. Ann NY Acad Sci. 2007;1095:504–512. - PubMed
-
- Kellof GJ, Crowell JA, Steele VE, Lubert RA, Malone WA, Boone CW, Koplovich L, Hawk ET, Liberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:S467–S471. - PubMed
-
- Friedl P, Wolf K. Tumor-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

