Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors
- PMID: 22246631
- PMCID: PMC3320034
- DOI: 10.4049/jimmunol.1101098
Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors
Abstract
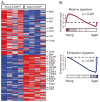
Aging is associated with suboptimal CD8 T cell responses to viral infections. It is not clear whether these poor responses are due to environmental influences or quantitative and qualitative changes in the pool of responding CD8 T cells. Our studies demonstrated several deleterious age-related changes in the pool of Ag-specific CD8 T cells that respond to infection. The majority of CD8 T cells from uninfected aged mice was CD44(Hi) and had increased expression of inhibitory receptors including PD1, LAG3, 2B4, and CD160. These aged CD44(Hi) CD8 T cells were transcriptionally similar to exhausted CD8 T cells found during chronic infections. In addition, the number of virus-specific precursors in aged mice prior to infection was decreased up to 10-fold, and many of these Ag-specific precursors had high expression of CD44 and PD1. Finally, TCR transgenic studies demonstrated that the CD44(Hi) Ag-specific CD8 T cells from unimmunized aged and young mice were qualitatively inferior compared with CD44(Lo) CD8 T cells from aged or young donors. Thus, a decrease in precursor frequency as well as qualitative changes of CD8 T cells during aging are directly related to impaired immunity.
Figures
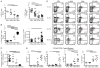


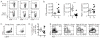

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

