Biomolecular chemistry of isopropyl fibrates
- PMID: 22246648
- PMCID: PMC3350796
- DOI: 10.1002/jps.23040
Biomolecular chemistry of isopropyl fibrates
Abstract
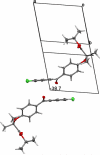
Isopropyl 2-[4-(4-chlorobenzoyl)-phenoxy]-2-methylpropanoic acid and isopropyl 2-(4-chlorophenoxy)-2-methylpropanoate, also known as fenofibrate and isopropyl (iPr) clofibrate, are hypolipidemic agents of the fibrate family. In a previously reported triclinic structure of fenofibrate (polymorph I), the methyl groups of the iPr moiety are located symmetrically about the carboxylate group. We report a new monoclinic form (polymorph II) of fenofibrate and a first structural description of iPr clofibrate, and in these the methyl groups are placed asymmetrically about the carboxylate group. In particular, the dihedral (torsion) angle between the hydrogen atom on the secondary C and the C atom of the carboxyl group makes a 2.74° angle about the ester O···C bond in the symmetric fenofibrate structure of polymorph I, whereas the same dihedral angle is 45.94° in polymorph II and -30.9° in the crystal structure of iPr clofibrate. Gas-phase density functional theory (DFT) geometry minimizations of fenofibrate and iPr clofibrate result in lowest energy conformations for both molecules with a value of about ±30° for this same angle between the OC-O-C plane and the C-H bond of the iPr group. A survey of crystal structures containing an iPr ester group reveals that the asymmetric conformation is predominant. Although the hydrogen atom on the secondary C atom of the iPr group is located at a comparable distance from the carbonyl oxygen in the symmetric and asymmetric fenofibrate (2.52 and 2.28 Å) and the iPr clofibrate (2.36 Å) structures, this hydrogen atom participates in a puckered five-membered ring arrangement in the latter two that is unlike the planar arrangement found in symmetric fenofibrate (polymorph I). Polar molecular surface area values indicate fenofibrate and iPr clofibrate are less able to act as acceptors of hydrogen bonds than their corresponding acid derivatives. Surface area calculations show that dynamic polar molecular surface area values of the iPr esters of the fibrates are lower than those of their acids, implying that the fibrates have better membrane permeability and a higher absorbability and hence are better prodrugs when these agents need to be orally administered.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


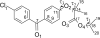




Similar articles
-
Synthesis of highly water-soluble fibrate derivatives via BGLation.Bioorg Med Chem Lett. 2012 Oct 15;22(20):6425-8. doi: 10.1016/j.bmcl.2012.08.057. Epub 2012 Aug 23. Bioorg Med Chem Lett. 2012. PMID: 22975299
-
Effects of fibric acid derivatives on biliary lipid composition.Am J Med. 1987 Nov 27;83(5B):37-43. doi: 10.1016/0002-9343(87)90869-2. Am J Med. 1987. PMID: 3318452 Review.
-
Potential use of fenofibrate and other fibric acid derivatives in the clinic.Am J Med. 1987 Nov 27;83(5B):85-9. doi: 10.1016/0002-9343(87)90876-x. Am J Med. 1987. PMID: 3688012
-
Development of Fibrates as Important Scaffolds in Medicinal Chemistry.ChemMedChem. 2019 Jun 5;14(11):1051-1066. doi: 10.1002/cmdc.201900128. Epub 2019 May 7. ChemMedChem. 2019. PMID: 30957432 Review.
-
Modulation of lipoprotein production in Hep G2 cells by fenofibrate and clofibrate.Biochem Pharmacol. 1992 Feb 4;43(3):625-33. doi: 10.1016/0006-2952(92)90586-8. Biochem Pharmacol. 1992. PMID: 1311585
Cited by
-
Formation of Organic Molecular Nanocrystals under Rigid Confinement with Analysis by Solid State NMR.CrystEngComm. 2014 Oct 21;16(39):9345-9352. doi: 10.1039/C4CE01087F. CrystEngComm. 2014. PMID: 25258590 Free PMC article.
-
Use of fibrates in the metabolic syndrome: A review.World J Diabetes. 2016 Mar 10;7(5):74-88. doi: 10.4239/wjd.v7.i5.74. World J Diabetes. 2016. PMID: 26981181 Free PMC article. Review.
-
Molecular dynamics of fibric acids.Eur J Chem. 2022 Jun;13(2):186-195. doi: 10.5155/eurjchem.13.2.186-195.2275. Epub 2022 Jun 30. Eur J Chem. 2022. PMID: 35991691 Free PMC article.
-
Towards controlling the crystallisation behaviour of fenofibrate melt: triggers of crystallisation and polymorphic transformation.RSC Adv. 2018 Apr 10;8(24):13513-13525. doi: 10.1039/c8ra01182f. eCollection 2018 Apr 9. RSC Adv. 2018. PMID: 35542519 Free PMC article.
-
Characterization of WY 14,643 and its Complex with Aldose Reductase.Sci Rep. 2016 Oct 10;6:34394. doi: 10.1038/srep34394. Sci Rep. 2016. PMID: 27721416 Free PMC article.
References
-
- Throp JM. Experimental evaluation of an orally active combination of androsterone with ethyl chlorophenoxy-isobutyrate. Lancet. 1962;1:1323–1326. - PubMed
-
- Miller DB, Spence JD. Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet. 1998;34(2):155–162. - PubMed
-
- Forcheron F, Cachefo A, Thevenon S, Pinteur C, Beylot M. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes. 2002;51(12):3486–3491. - PubMed
-
- Streel B, Hubert P, Ceccato A. Determination of fenofibric acid in human plasma using automated solid-phase extraction coupled to liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;742(2):391–400. - PubMed
-
- Thomas J, Bramlett KS, Montrose C, Foxworthy P, Eacho PI, McCann D, Cao G, Kiefer A, McCowan J, Yu KL, Grese T, Chin WW, Burris TP, Michael LF. A chemical switch regulates fibrate specificity for peroxisome proliferator-activated receptor alpha (PPARalpha) versus liver X receptor. J Biol Chem. 2003;278(4):2403–2410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

