Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis
- PMID: 22246864
- PMCID: PMC4854311
- DOI: 10.1165/rcmb.2011-0040OC
Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis
Abstract
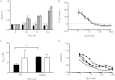
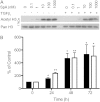
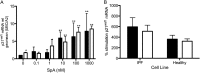
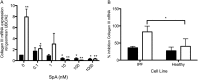
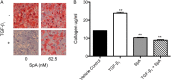
Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disorder characterized by the proliferation of interstitial fibroblasts and the deposition of extracellular matrix causing impaired gas exchange. Spiruchostatin A (SpA) is a histone deacetylase inhibitor (HDI) with selectivity toward Class I enzymes, which distinguishes it from other nonspecific HDIs that are reported to inhibit (myo)fibroblast proliferation and differentiation. Because the selectivity of HDIs may be important clinically, we postulated that SpA inhibits the proliferation and differentiation of IPF fibroblasts. Primary fibroblasts were grown from lung biopsy explants obtained from patients with IPF or from normal control subjects, using two-dimensional or three-dimensional culture models. The effect of SpA on fibroproliferation in serum-containing medium ± transforming growth factor (TGF)-β(1) was quantified by methylene blue binding. The acetylation of histone H3, the expression of the cell-cycle inhibitor p21(waf1), and the myofibroblast markers α-smooth muscle actin (α-SMA) and collagens I and III were determined by Western blotting, quantitative RT-PCR, immunofluorescent staining, or colorimetry. SpA inhibited the proliferation of IPF or normal fibroblasts in a time-dependent and concentration-dependent manner (concentration required to achieve 50% inhibition = 3.8 ± 0.4 nM versus 7.8 ± 0.2 nM, respectively; P < 0.05), with little cytotoxicity. Western blot analyses revealed that SpA caused a concentration-dependent increase in histone H3 acetylation, paralleling its antiproliferative effect. SpA also increased p21(waf1) expression, suggesting that direct cell-cycle regulation was the mechanism of inhibiting proliferation. Although treatment with TGF-β(1) induced myofibroblast differentiation associated with increased expression of α-SMA, collagen I and collagen III and soluble collagen release, these responses were potently inhibited by SpA. These data support the concept that bicyclic tetrapeptide HDIs merit further investigation as potential treatments for IPF.
Figures

References
-
- Valeyre D, Freynet O, Dion G, Bouvry D, Annesi-Maesano I, Nunes H. Epidemiology of interstitial lung diseases. Presse Med 2010;39:53–59. - PubMed
-
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. - PubMed
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810–816. - PubMed
-
- King TE, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

