Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction
- PMID: 22247250
- PMCID: PMC3289554
- DOI: 10.1105/tpc.111.092411
Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction
Abstract
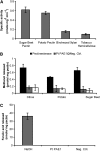
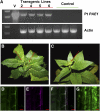
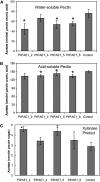
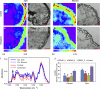
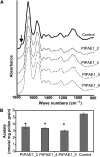
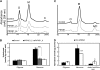
Pectin is a major component of the primary cell wall of higher plants. Some galacturonyl residues in the backbone of pectinaceous polysaccharides are often O-acetylated at the C-2 or C-3 position, and the resulting acetylesters change dynamically during the growth and development of plants. The processes involve both enzymatic acetylation and deacetylation. Through genomic sequence analysis, we identified a pectin acetylesterase (PAE1) from black cottonwood (Populus trichocarpa). Recombinant Pt PAE1 exhibited preferential activity in releasing the acetate moiety from sugar beet (Beta vulgaris) and potato (Solanum tuberosum) pectin in vitro. Overexpressing Pt PAE1 in tobacco (Nicotiana tabacum) decreased the level of acetyl esters of pectin but not of xylan. Deacetylation engendered differential changes in the composition and/or structure of cell wall polysaccharides that subsequently impaired the cellular elongation of floral styles and filaments, the germination of pollen grains, and the growth of pollen tubes. Consequently, plants overexpressing PAE1 exhibited severe male sterility. Furthermore, in contrast to the conventional view, PAE1-mediated deacetylation substantially lowered the digestibility of pectin. Our data suggest that pectin acetylesterase functions as an important structural regulator in planta by modulating the precise status of pectin acetylation to affect the remodeling and physiochemical properties of the cell wall's polysaccharides, thereby affecting cell extensibility.
Figures



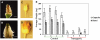
References
-
- André-Leroux G., Tessier D., Bonnin E. (2009). Endopolygalacturonases reveal molecular features for processivity pattern and tolerance towards acetylated pectin. Biochim. Biophys. Acta 1794: 5–13 - PubMed
-
- Benen J.A.E., Kester H.C.M., Visser J. (1999). Kinetic characterization of Aspergillus niger N400 endopolygalacturonases I, II and C. Eur. J. Biochem. 259: 577–585 - PubMed
-
- Biely P., MacKenzie C.R., Puls J., Schneider H. (1986). Cooperativity of esterases and xylanases in the enzymatic degradation of acetyl xylan. Biotechnol. 4: 731–733
-
- Bonnin E., Clavurier K., Daniel S., Kauppinen S., Mikkelsen J.D.M., Thibault J.F. (2008). Pectin acetylesterases from Aspergillus are able to deacetyalte homogalacturonan as well as rhamnogalacturonan. Carbohydr. Polym. 74: 411–418
-
- Bonnin E., Le Goff A., van Alebeek G.J.W.M., Voragen A.G.J., Thibault J.F. (2003). Mode of action of Fusarium moniliforme endopolygalacturonase towards acetylated pectin. Carbohydr. Polym. 52: 381–388
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

