Isoprenoid biosynthesis is required for miRNA function and affects membrane association of ARGONAUTE 1 in Arabidopsis
- PMID: 22247288
- PMCID: PMC3277166
- DOI: 10.1073/pnas.1112500109
Isoprenoid biosynthesis is required for miRNA function and affects membrane association of ARGONAUTE 1 in Arabidopsis
Erratum in
-
Correction for Brodersen et al., Isoprenoid biosynthesis is required for miRNA function and affects membrane association of ARGONAUTE 1 in Arabidopsis.Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2554. doi: 10.1073/pnas.1506636112. Epub 2015 Apr 16. Proc Natl Acad Sci U S A. 2015. PMID: 25883265 Free PMC article. No abstract available.
Abstract
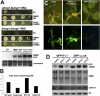
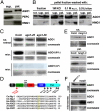
Plant and metazoan microRNAs (miRNAs) guide ARGONAUTE (AGO) protein complexes to regulate expression of complementary RNAs via base pairing. In the plant Arabidopsis thaliana, the main miRNA effector is AGO1, but few other factors required for miRNA activity are known. Here, we isolate the genes defined by the previously described miRNA action deficient (mad) mutants, mad3 and mad4. Both genes encode enzymes involved in isoprenoid biosynthesis. MAD3 encodes 3-hydroxy-3-methylglutaryl CoA reductase (HMG1), which functions in the initial C(5) building block biogenesis that precedes isoprenoid metabolism. HMG1 is a key regulatory enzyme that controls the amounts of isoprenoid end products. MAD4 encodes sterol C-8 isomerase (HYDRA1) that acts downstream in dedicated sterol biosynthesis. Using yeast complementation assays and in planta application of lovastatin, a competitive inhibitor of HMG1, we show that defects in HMG1 catalytic activity are sufficient to inhibit miRNA activity. Many isoprenoid derivatives are indispensable structural and signaling components, and especially sterols are essential membrane constituents. Accordingly, we provide evidence that AGO1 is a peripheral membrane protein. Moreover, specific hypomorphic mutant alleles of AGO1 display compromised membrane association and AGO1-membrane interaction is reduced upon knockdown of HMG1/MAD3. These results suggest a possible basis for the requirement of isoprenoid biosynthesis for the activity of plant miRNAs, and unravel mechanistic features shared with their metazoan counterparts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

