Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction
- PMID: 22247289
- PMCID: PMC3268296
- DOI: 10.1073/pnas.1112648109
Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction
Abstract
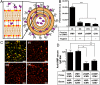
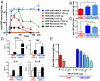
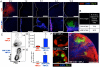
For subunit vaccines, adjuvants play a key role in shaping immunological memory. Nanoparticle (NP) delivery systems for antigens and/or molecular danger signals are promising adjuvants capable of promoting both cellular and humoral immune responses, but in most cases the mechanisms of action of these materials are poorly understood. Here, we studied the immune response elicited by NPs composed of multilamellar "stapled" lipid vesicles carrying a recombinant Plasmodium vivax circumsporozoite antigen, VMP001, both entrapped in the aqueous core and anchored to the lipid bilayer surfaces. Immunization with these particles and monophosphoryl lipid A (MPLA), a US Food and Drug Administration-approved immunostimulatory agonist for Toll-like receptor-4, promoted high-titer, high-avidity antibody responses against VMP001, lasting more than 1 y in mice at 10-fold lower doses than conventional adjuvants. Compared to soluble VMP001 mixed with MPLA, VMP001-NPs promoted broader humoral responses, targeting multiple epitopes of the protein and a more balanced Th1/Th2 cytokine profile from antigen-specific T cells. To begin to understand the underlying mechanisms, we examined components of the B-cell response and found that NPs promoted robust germinal center (GC) formation at low doses of antigen where no GC induction occurred with soluble protein immunization, and that GCs nucleated near depots of NPs accumulating in the draining lymph nodes over time. In parallel, NP vaccination enhanced the expansion of antigen-specific follicular helper T cells (T(fh)), compared to vaccinations with soluble VMP001 or alum. Thus, NP vaccines may be a promising strategy to enhance the durability, breadth, and potency of humoral immunity by enhancing key elements of the B-cell response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Reorienting our view of particle-based adjuvants for subunit vaccines.Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):999-1000. doi: 10.1073/pnas.1120993109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22308523 Free PMC article. No abstract available.
References
-
- Mueller I, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. - PubMed
-
- Nussenzweig V, Nussenzweig RS. Circumsporozoite proteins of malaria parasites. Cell. 1985;42:401–403. - PubMed
-
- Ballou WR. The development of the RTS,S malaria vaccine candidate: Challenges and lessons. Parasite Immunol. 2009;31:492–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

