Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis
- PMID: 22247470
- PMCID: PMC3317433
- DOI: 10.1167/iovs.11-8377
Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis
Abstract
Purpose: To test whether retinal pigment epithelial (RPE) cells are able to induce myeloid-derived suppressor cell (MDSC) differentiation from bone marrow (BM) progenitors.
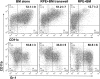
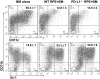
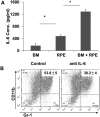
Methods: BM cells were cocultured with or without RPE cells in the presence of GM-CSF and IL-4. Numbers of resultant MDSCs were assessed by flow cytometry after 6 days of incubation. The ability of the RPE cell-induced MDSCs to inhibit T cells was evaluated by a CFSE-based T-cell proliferation assay. To explore the mechanism by which RPE cells induce MDSC differentiation, PD-L1-deficient RPE cells and blocking antibodies against TGF-β, CTLA-2α, and IL-6 were used. RPE cell-induced MDSCs were adoptively transferred into mice immunized with interphotoreceptor retinoid-binding protein in complete Freund's adjuvant to test their efficacy in suppressing autoreactive T-cell responses in experimental autoimmune uveitis (EAU).
Results: RPE cells induced the differentiation of MDSCs. These RPE cell-induced MDSCs significantly inhibited T-cell proliferation in a dose-dependent manner. PD-L1-deficient RPE cells induced MDSC differentiation as efficiently as wild-type RPE cells, and neutralizing TGF-β or CTLA-2α did not alter the numbers of induced MDSCs. However, blocking IL-6 reduced the efficacy of RPE cell-induced MDSC differentiation. Finally, adoptive transfer of RPE cell-induced MDSCs suppressed IRBP-specific T-cell responses that led to EAU.
Conclusions: RPE cells induce the differentiation of MDSCs from bone marrow progenitors. Both cell surface molecules and soluble factors are important in inducing MDSC differentiation. PD-L1, TGF-β, and CTLA-2α were not measurably involved in RPE cell-induced MDSC differentiation, whereas IL-6 was important in the process. The induction of MDSCs could be another mechanism by which RPE cells control immune reactions in the retina, and RPE cell-induced MDSCs should be further investigated as a potential approach to therapy for autoimmune posterior uveitis.
Figures



References
-
- Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47:100–105 - PubMed
-
- Buessow SC, Paul RD, Lopez DM. Influence of mammary tumor progression on phenotype and function of spleen and in situ lymphocytes in mice. J Natl Cancer Inst. 1984;73:249–255 - PubMed
-
- Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

