Generation of an inbred miniature pig model of retinitis pigmentosa
- PMID: 22247487
- PMCID: PMC3292381
- DOI: 10.1167/iovs.11-8784
Generation of an inbred miniature pig model of retinitis pigmentosa
Abstract
Purpose: The Pro23His (P23H) rhodopsin (RHO) mutation underlies the most common form of human autosomal dominant retinitis pigmentosa (adRP). The objective of this investigation was to establish a transgenic miniature swine model of RP using the human P23H RHO gene.
Methods: Somatic cell nuclear transfer (SCNT) was used to create transgenic miniature pigs that expressed the human P23H RHO mutation. From these experiments, six transgenic founders were identified whose retinal function was studied with full-field electroretinography (ffERG) from 3 months through 2 years. Progeny from one founder were generated and genotyped to determine transgene inheritance pattern. Retinal mRNA was isolated, and the ratio of P23H to wild-type pig RHO was measured.
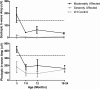
Results: A single transgene integration site was observed for five of the six founders. All founders had abnormal scotopic and photopic ffERGs after 3 months. The severity of the ffERG phenotype was grouped into moderately and severely affected groups. Offspring of one founder inherited the transgene as an autosomal dominant mutation. mRNA analyses demonstrated that approximately 80% of total RHO was mutant P23H.
Conclusions: Expression of the human RHO P23H transgene in the retina creates a miniature swine model with an inheritance pattern and retinal function that mimics adRP. This large-animal model can serve as a novel tool for the study of the pathogenesis and therapeutic intervention in the most common form of adRP.
Figures





Comment in
-
New tool for retinal degeneration research.Invest Ophthalmol Vis Sci. 2012 Feb 1;53(2):926. doi: 10.1167/iovs.12-9663. Invest Ophthalmol Vis Sci. 2012. PMID: 22345364 No abstract available.
References
-
- Inglehearn CF. Molecular genetics of human retinal dystrophies. Eye (Lond). 1998;12:571–579 - PubMed
-
- MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207 - PubMed
-
- Hendrickson A, Hicks D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res. 2002;74:435–444 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U42-RR18877/RR/NCRR NIH HHS/United States
- EY015128/EY/NEI NIH HHS/United States
- T32 HL076138/HL/NHLBI NIH HHS/United States
- EYO14800/PHS HHS/United States
- R01 EY015128/EY/NEI NIH HHS/United States
- R21 EY020647/EY/NEI NIH HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
- HL076138-08/HL/NHLBI NIH HHS/United States
- EY018608/EY/NEI NIH HHS/United States
- R01 EY002576/EY/NEI NIH HHS/United States
- EY02576/EY/NEI NIH HHS/United States
- EY-020647/EY/NEI NIH HHS/United States
- U42 RR018877/RR/NCRR NIH HHS/United States
- R01 EY018608/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

