White matter protection in congenital heart surgery
- PMID: 22247493
- PMCID: PMC3288390
- DOI: 10.1161/CIRCULATIONAHA.111.048215
White matter protection in congenital heart surgery
Abstract
Background: Neurodevelopmental delays in motor skills and white matter (WM) injury have been documented in congenital heart disease and after pediatric cardiac surgery. The lack of a suitable animal model has hampered our understanding of the cellular mechanisms underlying WM injury in these patients. Our aim is to identify an optimal surgical strategy for WM protection to reduce neurological injury in congenital heart disease patients.
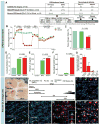
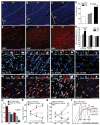
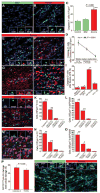
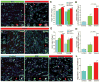
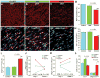
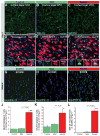
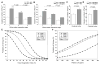
Methods and results: We developed a porcine cardiopulmonary bypass model that displays area-dependent WM maturation. In this model, WM injury was identified after cardiopulmonary bypass-induced ischemia-reperfusion injury. The degree of injury was inversely correlated with the maturation stage, which indicates maturation-dependent vulnerability of WM. Within different oligodendrocyte developmental stages, we show selective vulnerability of O4+ preoligodendrocytes, whereas oligodendrocyte progenitor cells were resistant to insults. This indicates that immature WM is vulnerable to cardiopulmonary bypass-induced injury but has an intrinsic potential for recovery mediated by endogenous oligodendrocyte progenitor cells. Oligodendrocyte progenitor cell number decreased with age, which suggests that earlier repair allows successful WM development. Oligodendrocyte progenitor cell proliferation was observed within a few days after cardiopulmonary bypass-induced ischemia-reperfusion injury; however, by 4 weeks, arrested oligodendrocyte maturation and delayed myelination were detected. Logistic model confirmed that maintenance of higher oxygenation and reduction of inflammation were effective in minimizing the risk of injury at immature stages of WM development.
Conclusions: Primary repair in neonates and young infants potentially provides successful WM development in congenital heart disease patients. Cardiac surgery during this susceptible period should avoid ischemia-reperfusion injury and minimize inflammation to prevent long-term WM-related neurological impairment.
Conflict of interest statement
Figures
Similar articles
-
Effects of preoperative hypoxia on white matter injury associated with cardiopulmonary bypass in a rodent hypoxic and brain slice model.Pediatr Res. 2014 May;75(5):618-25. doi: 10.1038/pr.2014.9. Epub 2014 Jan 31. Pediatr Res. 2014. PMID: 24488087 Free PMC article.
-
Rodent brain slice model for the study of white matter injury.J Thorac Cardiovasc Surg. 2013 Dec;146(6):1526-1533.e1. doi: 10.1016/j.jtcvs.2013.02.071. Epub 2013 Mar 27. J Thorac Cardiovasc Surg. 2013. PMID: 23540655 Free PMC article.
-
Microstructural Alterations and Oligodendrocyte Dysmaturation in White Matter After Cardiopulmonary Bypass in a Juvenile Porcine Model.J Am Heart Assoc. 2017 Aug 15;6(8):e005997. doi: 10.1161/JAHA.117.005997. J Am Heart Assoc. 2017. PMID: 28862938 Free PMC article.
-
Myocardial injury and protection related to cardiopulmonary bypass.Best Pract Res Clin Anaesthesiol. 2015 Jun;29(2):137-49. doi: 10.1016/j.bpa.2015.03.002. Epub 2015 Mar 27. Best Pract Res Clin Anaesthesiol. 2015. PMID: 26060026 Review.
-
Congenital cardiac anomalies and white matter injury.Trends Neurosci. 2015 Jun;38(6):353-63. doi: 10.1016/j.tins.2015.04.001. Epub 2015 May 1. Trends Neurosci. 2015. PMID: 25939892 Free PMC article. Review.
Cited by
-
Fractional anisotropy from diffusion tensor imaging correlates with acute astrocyte and myelin swelling in neonatal swine models of excitotoxic and hypoxic-ischemic brain injury.J Comp Neurol. 2021 Jul 1;529(10):2750-2770. doi: 10.1002/cne.25121. Epub 2021 Feb 15. J Comp Neurol. 2021. PMID: 33543493 Free PMC article.
-
Combining Hypothermia and Oleuropein Subacutely Protects Subcortical White Matter in a Swine Model of Neonatal Hypoxic-Ischemic Encephalopathy.J Neuropathol Exp Neurol. 2021 Jan 20;80(2):182-198. doi: 10.1093/jnen/nlaa132. J Neuropathol Exp Neurol. 2021. PMID: 33212486 Free PMC article.
-
Adult neurogenesis and the microbiota-gut-brain axis in farm animals: underestimated and understudied parameters for improving welfare in livestock farming.Front Neurosci. 2024 Nov 27;18:1493605. doi: 10.3389/fnins.2024.1493605. eCollection 2024. Front Neurosci. 2024. PMID: 39664450 Free PMC article. Review.
-
Mesenchymal Stromal Cell Delivery Via Cardiopulmonary Bypass Provides Neuroprotection in a Juvenile Porcine Model.JACC Basic Transl Sci. 2023 Sep 6;8(12):1521-1535. doi: 10.1016/j.jacbts.2023.07.002. eCollection 2023 Dec. JACC Basic Transl Sci. 2023. PMID: 38205346 Free PMC article.
-
Effects of preoperative hypoxia on white matter injury associated with cardiopulmonary bypass in a rodent hypoxic and brain slice model.Pediatr Res. 2014 May;75(5):618-25. doi: 10.1038/pr.2014.9. Epub 2014 Jan 31. Pediatr Res. 2014. PMID: 24488087 Free PMC article.
References
-
- Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. - PubMed
-
- Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. - PubMed
-
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease) Circulation. 2008;118:2395–2451. - PubMed
-
- Kaltman JR, Andropoulos DB, Checchia PA, Gaynor JW, Hoffman TM, Laussen PC, Ohye RG, Pearson GD, Pigula F, Tweddell J, Wernovsky G, Del Nido P. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation. 2010;121:2766–2772. - PubMed
-
- Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS045702/NS/NINDS NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- R01NS056427/NS/NINDS NIH HHS/United States
- P01NS0626860/NS/NINDS NIH HHS/United States
- P30HD40677/HD/NICHD NIH HHS/United States
- R01HL104173/HL/NHLBI NIH HHS/United States
- R01 NS056427/NS/NINDS NIH HHS/United States
- R01 HL060922/HL/NHLBI NIH HHS/United States
- R01 HL104173/HL/NHLBI NIH HHS/United States
- K08NS073793/NS/NINDS NIH HHS/United States
- K08 NS073793/NS/NINDS NIH HHS/United States
- R01 NS045702/NS/NINDS NIH HHS/United States
- R01HL060922/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

