AMP-forming acetyl coenzyme A synthetase in the outermost membrane of the hyperthermophilic crenarchaeon Ignicoccus hospitalis
- PMID: 22247508
- PMCID: PMC3294865
- DOI: 10.1128/JB.06130-11
AMP-forming acetyl coenzyme A synthetase in the outermost membrane of the hyperthermophilic crenarchaeon Ignicoccus hospitalis
Abstract
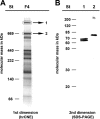
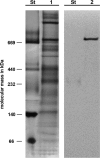
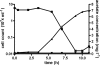
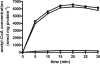
Ignicoccus hospitalis, a hyperthermophilic, chemolithoautotrophic crenarchaeon was found to possess a new CO(2) fixation pathway, the dicarboxylate/4-hydroxybutyrate cycle. The primary acceptor molecule for this pathway is acetyl coenzyme A (acetyl-CoA), which is regenerated in the cycle via the characteristic intermediate 4-hydroxybutyrate. In the presence of acetate, acetyl-CoA can alternatively be formed in a one-step mechanism via an AMP-forming acetyl-CoA synthetase (ACS). This enzyme was identified after membrane preparation by two-dimensional native PAGE/SDS-PAGE, followed by matrix-assisted laser desorption ionization-time of flight tandem mass spectrometry and N-terminal sequencing. The ACS of I. hospitalis exhibits a molecular mass of ∼690 kDa with a monomeric molecular mass of 77 kDa. Activity tests on isolated membranes and bioinformatic analyses indicated that the ACS is a constitutive membrane-associated (but not an integral) protein complex. Unexpectedly, immunolabeling on cells of I. hospitalis and other described Ignicoccus species revealed that the ACS is localized at the outermost membrane. This perfectly coincides with recent results that the ATP synthase and the H(2):sulfur oxidoreductase complexes are also located in the outermost membrane of I. hospitalis. These results imply that the intermembrane compartment of I. hospitalis is not only the site of ATP synthesis but may also be involved in the primary steps of CO(2) fixation.
Figures

Similar articles
-
The unusual cell biology of the hyperthermophilic Crenarchaeon Ignicoccus hospitalis.Antonie Van Leeuwenhoek. 2012 Aug;102(2):203-19. doi: 10.1007/s10482-012-9748-5. Epub 2012 Jun 1. Antonie Van Leeuwenhoek. 2012. PMID: 22653377 Review.
-
Insight into the proteome of the hyperthermophilic Crenarchaeon Ignicoccus hospitalis: the major cytosolic and membrane proteins.Arch Microbiol. 2008 Sep;190(3):379-94. doi: 10.1007/s00203-008-0399-x. Epub 2008 Jun 27. Arch Microbiol. 2008. PMID: 18584152 Free PMC article.
-
Purification of a Crenarchaeal ATP Synthase in the Light of the Unique Bioenergetics of Ignicoccus Species.J Bacteriol. 2019 Mar 13;201(7):e00510-18. doi: 10.1128/JB.00510-18. Print 2019 Apr 1. J Bacteriol. 2019. PMID: 30642991 Free PMC article.
-
Energized outer membrane and spatial separation of metabolic processes in the hyperthermophilic Archaeon Ignicoccus hospitalis.Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3152-6. doi: 10.1073/pnas.0911711107. Epub 2010 Feb 1. Proc Natl Acad Sci U S A. 2010. PMID: 20133662 Free PMC article.
-
Acetyl-coenzyme A synthetase (AMP forming).Cell Mol Life Sci. 2004 Aug;61(16):2020-30. doi: 10.1007/s00018-004-3448-x. Cell Mol Life Sci. 2004. PMID: 15316652 Free PMC article. Review.
Cited by
-
Functional compartmentalization and metabolic separation in a prokaryotic cell.Proc Natl Acad Sci U S A. 2021 Jun 22;118(25):e2022114118. doi: 10.1073/pnas.2022114118. Proc Natl Acad Sci U S A. 2021. PMID: 34161262 Free PMC article.
-
Life on the edge: functional genomic response of Ignicoccus hospitalis to the presence of Nanoarchaeum equitans.ISME J. 2015 Jan;9(1):101-14. doi: 10.1038/ismej.2014.112. Epub 2014 Jul 11. ISME J. 2015. PMID: 25012904 Free PMC article.
-
A Complex Endomembrane System in the Archaeon Ignicoccus hospitalis Tapped by Nanoarchaeum equitans.Front Microbiol. 2017 Jun 13;8:1072. doi: 10.3389/fmicb.2017.01072. eCollection 2017. Front Microbiol. 2017. PMID: 28659892 Free PMC article.
-
Cytochromes c in Archaea: distribution, maturation, cell architecture, and the special case of Ignicoccus hospitalis.Front Microbiol. 2015 May 12;6:439. doi: 10.3389/fmicb.2015.00439. eCollection 2015. Front Microbiol. 2015. PMID: 26029183 Free PMC article.
-
Acetyl-CoA synthetase activity is enzymatically regulated by lysine acetylation using acetyl-CoA or acetyl-phosphate as donor molecule.Nat Commun. 2024 Jul 17;15(1):6002. doi: 10.1038/s41467-024-49952-0. Nat Commun. 2024. PMID: 39019872 Free PMC article.
References
-
- Aceti DJ, Ferry JG. 1988. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J. Biol. Chem. 263:15444–15448 - PubMed
-
- Atteia A, et al. 2006. Pyruvate formate-lyase and a novel route of eukaryotic ATP synthesis in Chlamydomonas mitochondria. J. Biol. Chem. 281:9909–9918 - PubMed
-
- Berg IA, Ramos-Vera WH, Petri A, Huber H, Fuchs G. 2010. Study of the distribution of autotrophic carbon fixation cycles in Crenarchaeota. Microbiology 156:256–269 - PubMed
-
- Blum H, Beier H, Gross HJ. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
-
- Bräsen C, Schönheit P. 2001. Mechanisms of acetate formation and acetate activation in halophilic archaea. Arch. Microbiol. 175:360–368 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

