10'-Fluorovinblastine and 10'-Fluorovincristine: Synthesis of a Key Series of Modified Vinca Alkaloids
- PMID: 22247789
- PMCID: PMC3254105
- DOI: 10.1021/ml200236a
10'-Fluorovinblastine and 10'-Fluorovincristine: Synthesis of a Key Series of Modified Vinca Alkaloids
Abstract
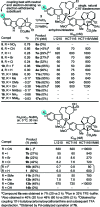
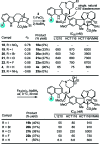
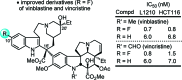
A study on the impact of catharanthine C10 and C12 indole substituents on the biomimetic Fe(III)-mediated coupling with vindoline led to the discovery and characterization of two new and substantially more potent derivatives, 10'-fluorovinblastine and 10'-fluorovincristine. In addition to defining a pronounced and unanticipated substituent effect on the biomimetic coupling, fluorine substitution at C10', which minimally alters the natural products, was found to uniquely enhance the activity 8-fold against both sensitive (IC(50) = 800 pM, HCT116) and vinblastine-resistant tumor cell lines (IC(50) = 80 nM, HCT166/VM46). As depicted in the X-ray structure of vinblastine bound to tubulin, this site resides at one end of the upper portion of the T-shaped conformation of the tubulin-bound molecule, suggesting the 10'-fluorine substituent makes critical contacts with the protein at a hydrophobic site uniquely sensitive to steric interactions.
Figures
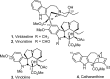

References
-
- Noble R. L.; Beer C. T.; Cutts J. H. Role of chance observations in chemotherapy: Vinca rosea. Ann. N.Y. Acad. Sci. 1958, 76, 882. - PubMed
-
- Noble R. L. Catharanthus roseus (Vinca rosea) - importance and value of a chance observation. Lloydia 1964, 27, 280.
-
- Svoboda G. H.; Nuess N.; Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J. Am. Pharm. Assoc. Sci. Ed. 1959, 48, 659. - PubMed
-
- Moncrief J. W.; Lipscomb W. N. Structures of leurocristine (vincristine) and vincaleukoblastine. X-ray analysis of leurocristine methiodide. J. Am. Chem. Soc. 1965, 84, 4963. - PubMed
-
- Neuss N.; Neuss M. N.. Therapeutic use of bisindole alkaloids from catharanthus. In The Alkaloids; Brossi A., Suffness M., Eds.; Academic: San Diego, 1990; Vol. 37, p 229.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

