Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge
- PMID: 22248083
- PMCID: PMC3283491
- DOI: 10.1186/1742-2094-9-9
Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge
Abstract
Background: There is ample evidence that psychological stress adversely affects many diseases. Recent evidence has shown that intense stressors can increase inflammation within the brain, a known mediator of many diseases. However, long-term outcomes of chronic psychological stressors that elicit a neuroinflammatory response remain unknown.
Methods: To address this, we have modified previously described models of rat/mouse predatory stress (PS) to increase the intensity of the interaction. We postulated that these modifications would enhance the predator-prey experience and increase neuroinflammation and behavioral dysfunction in prey animals. In addition, another group of mice were subjected to a modified version of chronic unpredictable stress (CUS), an often-used model of chronic stress that utilizes a combination of stressors that include physical, psychological, chemical, and other. The CUS model has been shown to exacerbate a number of inflammatory-related diseases via an unknown mechanism. Using these two models we sought to determine: 1) whether chronic PS or CUS modulated the inflammatory response as a proposed mechanism by which behavioral deficits might be mediated, and 2) whether chronic exposure to a pure psychological stressor (PS) leads to deficits similar to those produced by a CUS model containing psychological and physical stressors. Finally, to determine whether acute PS has neuroinflammatory consequences, adult mice were examined at various time-points after PS for changes in inflammation.
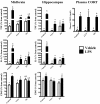
Results: Adolescent mice subjected to chronic PS had increased basal expression of inflammation within the midbrain. CUS and chronic PS mice also had an impaired inflammatory response to a subsequent lipopolysaccharide challenge and PS mice displayed increased anxiety- and depressive-like behaviors following chronic stress. Finally, adult mice subjected to acute predatory stress had increased gene expression of inflammatory factors.
Conclusion: Our results demonstrate that predatory stress, an ethologically relevant stressor, can elicit changes in neuroinflammation and behavior. The predatory stress model may be useful in elucidating mechanisms by which psychological stress modulates diseases with an inflammatory component.
Figures

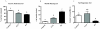


References
-
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of pediatrics & adolescent medicine. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

