Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells
- PMID: 22248145
- PMCID: PMC3273432
- DOI: 10.1186/1472-6882-12-6
Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells
Abstract
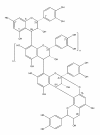
Background: Oral candidiasis is a common fungal disease mainly caused by Candida albicans. The aim of this study was to investigate the effects of A-type cranberry proanthocyanidins (AC-PACs) on pathogenic properties of C. albicans as well as on the inflammatory response of oral epithelial cells induced by this oral pathogen.
Methods: Microplate dilution assays were performed to determine the effect of AC-PACs on C. albicans growth as well as biofilm formation stained with crystal violet. Adhesion of FITC-labeled C. albicans to oral epithelial cells and to acrylic resin disks was monitored by fluorometry. The effects of AC-PACs on C. albicans-induced cytokine secretion, nuclear factor-kappa B (NF-κB) p65 activation and kinase phosphorylation in oral epithelial cells were determined by immunological assays.
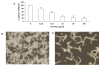
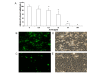
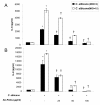
Results: Although AC-PACs did not affect growth of C. albicans, it prevented biofilm formation and reduced adherence of C. albicans to oral epithelial cells and saliva-coated acrylic resin discs. In addition, AC-PACs significantly decreased the secretion of IL-8 and IL-6 by oral epithelial cells stimulated with C. albicans. This anti-inflammatory effect was associated with reduced activation of NF-κB p65 and phosphorylation of specific signal intracellular kinases.
Conclusion: AC-PACs by affecting the adherence properties of C. albicans and attenuating the inflammatory response induced by this pathogen represent potential novel therapeutic agents for the prevention/treatment of oral candidiasis.
Figures
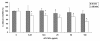
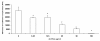

Similar articles
-
Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins.Antimicrob Agents Chemother. 2010 May;54(5):1778-84. doi: 10.1128/AAC.01432-09. Epub 2010 Feb 22. Antimicrob Agents Chemother. 2010. PMID: 20176905 Free PMC article.
-
Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm- and adherence-specific mechanisms.J Antimicrob Chemother. 2014 Feb;69(2):428-36. doi: 10.1093/jac/dkt398. Epub 2013 Oct 10. J Antimicrob Chemother. 2014. PMID: 24114570 Free PMC article.
-
Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells.BMC Complement Altern Med. 2019 Nov 8;19(1):303. doi: 10.1186/s12906-019-2730-2. BMC Complement Altern Med. 2019. PMID: 31703673 Free PMC article.
-
Cranberry proanthocyanidins: natural weapons against periodontal diseases.J Agric Food Chem. 2012 Jun 13;60(23):5728-35. doi: 10.1021/jf203304v. Epub 2011 Nov 29. J Agric Food Chem. 2012. PMID: 22082264 Review.
-
Phytomedicines for Candida-associated denture stomatitis.Fitoterapia. 2010 Jul;81(5):323-8. doi: 10.1016/j.fitote.2009.12.003. Epub 2009 Dec 16. Fitoterapia. 2010. PMID: 20026192 Review.
Cited by
-
Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens.Molecules. 2020 Aug 17;25(16):3748. doi: 10.3390/molecules25163748. Molecules. 2020. PMID: 32824589 Free PMC article. Review.
-
In Vitro Antifungal and Antivirulence Activities of Biologically Synthesized Ethanolic Extract of Propolis-Loaded PLGA Nanoparticles against Candida albicans.Evid Based Complement Alternat Med. 2019 Nov 30;2019:3715481. doi: 10.1155/2019/3715481. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31871479 Free PMC article.
-
Cranberry-derived proanthocyanidins induce a differential transcriptomic response within Candida albicans urinary biofilms.PLoS One. 2018 Aug 8;13(8):e0201969. doi: 10.1371/journal.pone.0201969. eCollection 2018. PLoS One. 2018. PMID: 30089157 Free PMC article.
-
Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus.Sci Rep. 2018 Dec 6;8(1):17696. doi: 10.1038/s41598-018-35793-7. Sci Rep. 2018. PMID: 30523307 Free PMC article.
-
Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections.Asian Pac J Trop Biomed. 2014 Feb;4(2):104-8. doi: 10.1016/S2221-1691(14)60217-3. Asian Pac J Trop Biomed. 2014. PMID: 25182280 Free PMC article.
References
-
- Zunt SL. Oral candidiasis: Diagnosis and treatment. J Pract Hyg. 2000;9:31–36.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

