The roles of Rhodobacter sphaeroides copper chaperones PCu(A)C and Sco (PrrC) in the assembly of the copper centers of the aa(3)-type and the cbb(3)-type cytochrome c oxidases
- PMID: 22248670
- PMCID: PMC3336013
- DOI: 10.1016/j.bbabio.2012.01.003
The roles of Rhodobacter sphaeroides copper chaperones PCu(A)C and Sco (PrrC) in the assembly of the copper centers of the aa(3)-type and the cbb(3)-type cytochrome c oxidases
Abstract
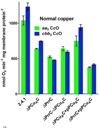
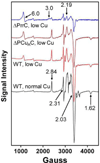
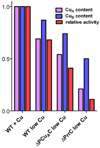
The α proteobacter Rhodobacter sphaeroides accumulates two cytochrome c oxidases (CcO) in its cytoplasmic membrane during aerobic growth: a mitochondrial-like aa(3)-type CcO containing a di-copper Cu(A) center and mono-copper Cu(B), plus a cbb(3)-type CcO that contains Cu(B) but lacks Cu(A). Three copper chaperones are located in the periplasm of R. sphaeroides, PCu(A)C, PrrC (Sco) and Cox11. Cox11 is required to assemble Cu(B) of the aa(3)-type but not the cbb(3)-type CcO. PrrC is homologous to mitochondrial Sco1; Sco proteins are implicated in Cu(A) assembly in mitochondria and bacteria, and with Cu(B) assembly of the cbb(3)-type CcO. PCu(A)C is present in many bacteria, but not mitochondria. PCu(A)C of Thermus thermophilus metallates a Cu(A) center in vitro, but its in vivo function has not been explored. Here, the extent of copper center assembly in the aa(3)- and cbb(3)-type CcOs of R. sphaeroides has been examined in strains lacking PCu(A)C, PrrC, or both. The absence of either chaperone strongly lowers the accumulation of both CcOs in the cells grown in low concentrations of Cu(2+). The absence of PrrC has a greater effect than the absence of PCu(A)C and PCu(A)C appears to function upstream of PrrC. Analysis of purified aa(3)-type CcO shows that PrrC has a greater effect on the assembly of its Cu(A) than does PCu(A)C, and both chaperones have a lesser but significant effect on the assembly of its Cu(B) even though Cox11 is present. Scenarios for the cellular roles of PCu(A)C and PrrC are considered. The results are most consistent with a role for PrrC in the capture and delivery of copper to Cu(A) of the aa(3)-type CcO and to Cu(B) of the cbb(3)-type CcO, while the predominant role of PCu(A)C may be to capture and deliver copper to PrrC and Cox11. This article is part of a Special Issue entitled: Biogenesis/Assembly of Respiratory Enzyme Complexes.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Mutagenic analysis of Cox11 of Rhodobacter sphaeroides: insights into the assembly of Cu(B) of cytochrome c oxidase.Biochemistry. 2010 Jul 13;49(27):5651-61. doi: 10.1021/bi1003876. Biochemistry. 2010. PMID: 20524628 Free PMC article.
-
From redox flow to gene regulation: role of the PrrC protein of Rhodobacter sphaeroides 2.4.1.Biochemistry. 2000 Feb 29;39(8):2052-62. doi: 10.1021/bi9923858. Biochemistry. 2000. PMID: 10684655
-
The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb₃-type cytochrome oxidase in Rhodobacter capsulatus.Biochim Biophys Acta. 2012 Nov;1817(11):2005-15. doi: 10.1016/j.bbabio.2012.06.621. Epub 2012 Jul 4. Biochim Biophys Acta. 2012. PMID: 22771512 Free PMC article.
-
Biogenesis of cbb(3)-type cytochrome c oxidase in Rhodobacter capsulatus.Biochim Biophys Acta. 2012 Jun;1817(6):898-910. doi: 10.1016/j.bbabio.2011.10.011. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22079199 Free PMC article. Review.
-
Assembly factors and ATP-dependent proteases in cytochrome c oxidase biogenesis.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1149-58. doi: 10.1016/j.bbabio.2010.04.006. Epub 2010 Apr 14. Biochim Biophys Acta. 2010. PMID: 20398622 Review.
Cited by
-
PCuAC domains from methane-oxidizing bacteria use a histidine brace to bind copper.J Biol Chem. 2019 Nov 1;294(44):16351-16363. doi: 10.1074/jbc.RA119.010093. Epub 2019 Sep 16. J Biol Chem. 2019. PMID: 31527086 Free PMC article.
-
Characterization of the PIB-Type ATPases present in Thermus thermophilus.J Bacteriol. 2012 Aug;194(15):4107-13. doi: 10.1128/JB.00849-12. Epub 2012 May 25. J Bacteriol. 2012. PMID: 22636781 Free PMC article.
-
Morphological development and cytochrome c oxidase activity in Streptomyces lividans are dependent on the action of a copper bound Sco protein.Open Biol. 2013 Jan 23;3(1):120163. doi: 10.1098/rsob.120163. Open Biol. 2013. PMID: 23345541 Free PMC article.
-
Bioinformatic Exploration of Metal-Binding Proteome of Zoonotic Pathogen Orientia tsutsugamushi.Front Genet. 2019 Sep 24;10:797. doi: 10.3389/fgene.2019.00797. eCollection 2019. Front Genet. 2019. PMID: 31608099 Free PMC article.
-
Oxygen Reductases in Alphaproteobacterial Genomes: Physiological Evolution From Low to High Oxygen Environments.Front Microbiol. 2019 Mar 18;10:499. doi: 10.3389/fmicb.2019.00499. eCollection 2019. Front Microbiol. 2019. PMID: 30936856 Free PMC article.
References
-
- Hosler JP, Fetter J, Tecklenburg MM, Espe M, Lerma C, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping, and spectral analysis. J. Biol. Chem. 1992;267:24264–24272. - PubMed
-
- Cao J, Hosler J, Shapleigh J, Revzin A, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. The coxII/coxIII operon codes for structural and assembly proteins homologous to those in yeast. J. Biol. Chem. 1992;267:24273–24278. - PubMed
-
- Shapleigh JP, Gennis RB. Cloning, sequencing, and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol. Microbiol. 1992;6:635–642. - PubMed
-
- Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J. Mol. Biol. 2002;321:329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

