Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance
- PMID: 22248732
- PMCID: PMC3307098
- DOI: 10.1016/j.bcp.2012.01.002
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance
Abstract
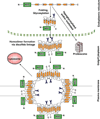
Since cloning of the ATP-binding cassette (ABC) family member breast cancer resistance protein (BCRP/ABCG2) and its characterization as a multidrug resistance efflux transporter in 1998, BCRP has been the subject of more than two thousand scholarly articles. In normal tissues, BCRP functions as a defense mechanism against toxins and xenobiotics, with expression in the gut, bile canaliculi, placenta, blood-testis and blood-brain barriers facilitating excretion and limiting absorption of potentially toxic substrate molecules, including many cancer chemotherapeutic drugs. BCRP also plays a key role in heme and folate homeostasis, which may help normal cells survive under conditions of hypoxia. BCRP expression appears to be a characteristic of certain normal tissue stem cells termed "side population cells," which are identified on flow cytometric analysis by their ability to exclude Hoechst 33342, a BCRP substrate fluorescent dye. Hence, BCRP expression may contribute to the natural resistance and longevity of these normal stem cells. Malignant tissues can exploit the properties of BCRP to survive hypoxia and to evade exposure to chemotherapeutic drugs. Evidence is mounting that many cancers display subpopulations of stem cells that are responsible for tumor self-renewal. Such stem cells frequently manifest the "side population" phenotype characterized by expression of BCRP and other ABC transporters. Along with other factors, these transporters may contribute to the inherent resistance of these neoplasms and their failure to be cured.
Published by Elsevier Inc.
Figures

References
-
- Ross DD, Nakanishi T. Impact of breast cancer resistance protein on cancer treatment outcomes. In: Zhou J, editor. Methods in Molecular Biology. New York: Humana Press, Springer; 2010. pp. 251–290. - PubMed
-
- Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

