Inactivation of thiol-dependent enzymes by hypothiocyanous acid: role of sulfenyl thiocyanate and sulfenic acid intermediates
- PMID: 22248862
- PMCID: PMC3523338
- DOI: 10.1016/j.freeradbiomed.2011.12.024
Inactivation of thiol-dependent enzymes by hypothiocyanous acid: role of sulfenyl thiocyanate and sulfenic acid intermediates
Abstract
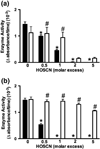
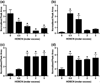
Myeloperoxidase (MPO) forms reactive oxidants including hypochlorous and hypothiocyanous acids (HOCl and HOSCN) under inflammatory conditions. HOCl causes extensive tissue damage and plays a role in the progression of many inflammatory-based diseases. Although HOSCN is a major MPO oxidant, particularly in smokers, who have elevated plasma thiocyanate, the role of this oxidant in disease is poorly characterized. HOSCN induces cellular damage by targeting thiols. However, the specific targets and mechanisms involved in this process are not well defined. We show that exposure of macrophages to HOSCN results in the inactivation of intracellular enzymes, including creatine kinase (CK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In each case, the active-site thiol residue is particularly sensitive to oxidation, with evidence for reversible inactivation and the formation of sulfenyl thiocyanate and sulfenic acid intermediates, on treatment with HOSCN (less than fivefold molar excess). Experiments with DAz-2, a cell-permeable chemical trap for sulfenic acids, demonstrate that these intermediates are formed on many cellular proteins, including GAPDH and CK, in macrophages exposed to HOSCN. This is the first direct evidence for the formation of protein sulfenic acids in HOSCN-treated cells and highlights the potential of this oxidant to perturb redox signaling processes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures







Similar articles
-
Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells.Free Radic Biol Med. 2013 Dec;65:1352-1362. doi: 10.1016/j.freeradbiomed.2013.10.007. Epub 2013 Oct 10. Free Radic Biol Med. 2013. PMID: 24120969
-
Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages.Free Radic Biol Med. 2016 May;94:88-98. doi: 10.1016/j.freeradbiomed.2016.02.016. Epub 2016 Feb 17. Free Radic Biol Med. 2016. PMID: 26898502
-
The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages.Biochem J. 2010 Aug 15;430(1):161-9. doi: 10.1042/BJ20100082. Biochem J. 2010. PMID: 20528774 Free PMC article.
-
Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids.Free Radic Res. 2012 Aug;46(8):975-95. doi: 10.3109/10715762.2012.667566. Epub 2012 Apr 23. Free Radic Res. 2012. PMID: 22348603 Review.
-
The therapeutic potential of thiocyanate and hypothiocyanous acid against pulmonary infections.Free Radic Biol Med. 2024 Jul;219:104-111. doi: 10.1016/j.freeradbiomed.2024.04.217. Epub 2024 Apr 11. Free Radic Biol Med. 2024. PMID: 38608822 Free PMC article. Review.
Cited by
-
Association between environmental exposure to perchlorate, nitrate, and thiocyanate and serum α-Klotho levels among adults from the National Health and nutrition examination survey (2007-2014).BMC Geriatr. 2022 Sep 12;22(1):740. doi: 10.1186/s12877-022-03444-2. BMC Geriatr. 2022. PMID: 36096772 Free PMC article.
-
The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host.Free Radic Biol Med. 2020 Jan;146:324-332. doi: 10.1016/j.freeradbiomed.2019.11.016. Epub 2019 Nov 15. Free Radic Biol Med. 2020. PMID: 31740228 Free PMC article.
-
The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens.Cell Mol Life Sci. 2021 Jan;78(2):385-414. doi: 10.1007/s00018-020-03591-y. Epub 2020 Jul 13. Cell Mol Life Sci. 2021. PMID: 32661559 Free PMC article. Review.
-
Redox regulation of protein damage in plasma.Redox Biol. 2014 Jan 20;2:430-5. doi: 10.1016/j.redox.2014.01.010. eCollection 2014. Redox Biol. 2014. PMID: 24624332 Free PMC article. Review.
-
Myeloperoxidase Modulates Hydrogen Peroxide Mediated Cellular Damage in Murine Macrophages.Antioxidants (Basel). 2020 Dec 10;9(12):1255. doi: 10.3390/antiox9121255. Antioxidants (Basel). 2020. PMID: 33321763 Free PMC article.
References
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. - PubMed
-
- Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:1199–1234. - PubMed
-
- Pruitt KM, Manssonrahemtulla B, Baldone DC, Rahemtulla F. Steady-state kinetics of thiocyanate oxidation catalyzed by human salivary peroxidase. Biochemistry. 1988;27:240–245. - PubMed
-
- Reiter B, Harnulv G. Lactoperoxidase antibacterial system—natural occurrence, biological functions and practical applications. J. Food Prot. 1984;47:724–732. - PubMed
-
- Slungaard A, Mahoney JR., Jr Thiocyanate is the major substrate for eosinophil peroxidase in physiologic fluids: implications for cytotoxicity. J. Biol. Chem. 1991;266:4903–4910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

