Synthesis and evaluation of potent and selective human V1a receptor antagonists as potential ligands for PET or SPECT imaging
- PMID: 22249122
- PMCID: PMC3449305
- DOI: 10.1016/j.bmc.2011.12.013
Synthesis and evaluation of potent and selective human V1a receptor antagonists as potential ligands for PET or SPECT imaging
Abstract
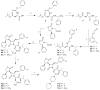
SRX246 is a potent, highly selective human vasopressin V1a antagonist that crosses the blood-brain barrier in rats. CNS penetration makes SRX246 an ideal candidate for potential radiolabeling and use in visualization and characterization of the role of the V1a receptor in multiple stress-related disorders. Before radiolabeling studies, cold reference analogs of SRX246 were prepared. This study describes the synthesis and in vitro screening for human V1a receptor binding and permeability of fluoro, iodo, and methyl reference compounds for SRX246 and the preparation of a tin precursor. For each compound, the potential utility of corresponding radiolabeled analogs for PET and SPECT imaging is discussed.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
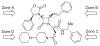
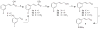
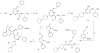
Similar articles
-
Pharmacokinetics and metabolism of SRX246: a potent and selective vasopressin 1a antagonist.J Pharm Sci. 2013 Jun;102(6):2033-2043. doi: 10.1002/jps.23495. Epub 2013 Mar 7. J Pharm Sci. 2013. PMID: 23471831
-
The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: a randomized proof-of-concept study.Psychopharmacology (Berl). 2021 Sep;238(9):2393-2403. doi: 10.1007/s00213-021-05861-4. Epub 2021 May 10. Psychopharmacology (Berl). 2021. PMID: 33970290 Free PMC article. Clinical Trial.
-
Preparation of highly potent and selective non-peptide antagonists of the arginine vasopressin V1A receptor by introduction of a 2-ethyl-1H-1-imidazolyl group.Chem Pharm Bull (Tokyo). 2005 Jul;53(7):764-9. doi: 10.1248/cpb.53.764. Chem Pharm Bull (Tokyo). 2005. PMID: 15997131
-
Vasopressin V1a and V1b receptor modulators: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jun;25(6):711-22. doi: 10.1517/13543776.2015.1026257. Epub 2015 Mar 16. Expert Opin Ther Pat. 2015. PMID: 25776143 Review.
-
Nonpeptide vasopressin receptor antagonists: development of selective and orally active V1a, V2 and V1b receptor ligands.Prog Brain Res. 2002;139:197-210. doi: 10.1016/s0079-6123(02)39017-4. Prog Brain Res. 2002. PMID: 12436936 Review.
Cited by
-
Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior.Front Neuroendocrinol. 2016 Jan;40:1-23. doi: 10.1016/j.yfrne.2015.04.003. Epub 2015 May 4. Front Neuroendocrinol. 2016. PMID: 25951955 Free PMC article. Review.
-
The Vasopressin 1a Receptor Antagonist SRX246 Reduces Aggressive Behavior in Huntington's Disease.J Pers Med. 2022 Sep 22;12(10):1561. doi: 10.3390/jpm12101561. J Pers Med. 2022. PMID: 36294700 Free PMC article.
-
Development of a triazolobenzodiazepine-based PET probe for subtype-selective vasopressin 1A receptor imaging.Pharmacol Res. 2021 Nov;173:105886. doi: 10.1016/j.phrs.2021.105886. Epub 2021 Sep 16. Pharmacol Res. 2021. PMID: 34536549 Free PMC article.
-
Development of a radioligand for imaging V1a vasopressin receptors with PET.Eur J Med Chem. 2017 Oct 20;139:644-656. doi: 10.1016/j.ejmech.2017.08.037. Epub 2017 Aug 18. Eur J Med Chem. 2017. PMID: 28843869 Free PMC article.
-
Positron Emission Tomography in the Neuroimaging of Autism Spectrum Disorder: A Review.Front Neurosci. 2022 Apr 13;16:806876. doi: 10.3389/fnins.2022.806876. eCollection 2022. Front Neurosci. 2022. PMID: 35495051 Free PMC article. Review.
References
-
- Ring RH. Curr Pharm Des. 2005;11:205. - PubMed
-
- Serradeil-Le Gal C, Wagnon J, Valette G, Garcia G, Pascal M, Maffrand JP, Le Fur G. Prog Brain Res. 2002;139:197. - PubMed
-
- Goekoop JG, de Winter RP, de Rijk R, Zwinderman KH, Frankhuijzen-Sierevogel A, Wiegant VM. Psychiatry Res. 2006;141:201. - PubMed
-
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M. Neurosci Biobehav Rev. 2007;31:89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

