Structural analysis of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular domain reveals a conserved interaction epitope
- PMID: 22249178
- PMCID: PMC3293552
- DOI: 10.1074/jbc.M111.330779
Structural analysis of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular domain reveals a conserved interaction epitope
Abstract
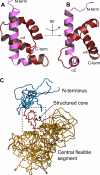
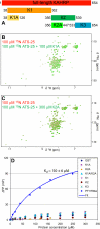
Plasmodium falciparum-infected red blood cells adhere to endothelial cells, thereby obstructing the microvasculature. Erythrocyte adherence is directly associated with severe malaria and increased disease lethality, and it is mediated by the PfEMP1 family. PfEMP1 clustering in knob-like protrusions on the erythrocyte membrane is critical for cytoadherence, however the molecular mechanisms behind this system remain elusive. Here, we show that the intracellular domains of the PfEMP1 family (ATS) share a unique molecular architecture, which comprises a minimal folded core and extensive flexible elements. A conserved flexible segment at the ATS center is minimally restrained by the folded core. Yeast-two-hybrid data and a novel sequence analysis method suggest that this central segment contains a conserved protein interaction epitope. Interestingly, ATS in solution fails to bind the parasite knob-associated histidine-rich protein (KAHRP), an essential cytoadherence component. Instead, we demonstrate that ATS associates with PFI1780w, a member of the Plasmodium helical interspersed sub-telomeric (PHIST) family. PHIST domains are widespread in exported parasite proteins, however this is the first specific molecular function assigned to any variant of this family. We propose that PHIST domains facilitate protein interactions, and that the conserved ATS epitope may be targeted to disrupt the parasite cytoadherence system.
Figures


Similar articles
-
A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface.FASEB J. 2014 Oct;28(10):4420-33. doi: 10.1096/fj.14-256057. Epub 2014 Jun 30. FASEB J. 2014. PMID: 24983468 Free PMC article.
-
Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions.PLoS Pathog. 2017 Aug 14;13(8):e1006552. doi: 10.1371/journal.ppat.1006552. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28806784 Free PMC article.
-
Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton.Mol Biochem Parasitol. 2000 May;108(2):237-47. doi: 10.1016/s0166-6851(00)00227-9. Mol Biochem Parasitol. 2000. PMID: 10838226
-
Plasmodium helical interspersed subtelomeric family-an enigmatic piece of the Plasmodium biology puzzle.Parasitol Res. 2019 Oct;118(10):2753-2766. doi: 10.1007/s00436-019-06420-9. Epub 2019 Aug 15. Parasitol Res. 2019. PMID: 31418110 Review.
-
The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research.Mol Biochem Parasitol. 2014 Jul;195(2):82-7. doi: 10.1016/j.molbiopara.2014.07.006. Epub 2014 Jul 23. Mol Biochem Parasitol. 2014. PMID: 25064606 Free PMC article. Review.
Cited by
-
Investigating a Plasmodium falciparum erythrocyte invasion phenotype switch at the whole transcriptome level.Sci Rep. 2020 Jan 14;10(1):245. doi: 10.1038/s41598-019-56386-y. Sci Rep. 2020. PMID: 31937828 Free PMC article.
-
Variable Surface Antigens of Plasmodium falciparum: Protein Families with Divergent Roles.Protein Pept Lett. 2024;31(6):409-423. doi: 10.2174/0109298665298567240530170924. Protein Pept Lett. 2024. PMID: 38910420 Review.
-
Protein-Specific Features Associated with Variability in Human Antibody Responses to Plasmodium falciparum Malaria Antigens.Am J Trop Med Hyg. 2018 Jan;98(1):57-66. doi: 10.4269/ajtmh.17-0437. Am J Trop Med Hyg. 2018. PMID: 29141757 Free PMC article.
-
Plasmodium falciparum-infected erythrocyte knob density is linked to the PfEMP1 variant expressed.mBio. 2015 Oct 6;6(5):e01456-15. doi: 10.1128/mBio.01456-15. mBio. 2015. PMID: 26443460 Free PMC article.
-
A member of the Plasmodium falciparum PHIST family binds to the erythrocyte cytoskeleton component band 4.1.Malar J. 2013 May 11;12:160. doi: 10.1186/1475-2875-12-160. Malar J. 2013. PMID: 23663475 Free PMC article.
References
-
- Haldar K., Mohandas N. (2007) Erythrocyte remodeling by malaria parasites. Curr. Opin. Hematol. 14, 203–209 - PubMed
-
- Maier A. G., Cooke B. M., Cowman A. F., Tilley L. (2009) Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 7, 341–354 - PubMed
-
- Kraemer S. M., Smith J. D. (2006) A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9, 374–380 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

