The Drosophila juvenile hormone receptor candidates methoprene-tolerant (MET) and germ cell-expressed (GCE) utilize a conserved LIXXL motif to bind the FTZ-F1 nuclear receptor
- PMID: 22249180
- PMCID: PMC3293574
- DOI: 10.1074/jbc.M111.327254
The Drosophila juvenile hormone receptor candidates methoprene-tolerant (MET) and germ cell-expressed (GCE) utilize a conserved LIXXL motif to bind the FTZ-F1 nuclear receptor
Abstract
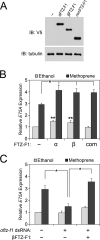
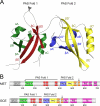
Juvenile hormone (JH) has been implicated in many developmental processes in holometabolous insects, but its mechanism of signaling remains controversial. We previously found that in Drosophila Schneider 2 cells, the nuclear receptor FTZ-F1 is required for activation of the E75A gene by JH. Here, we utilized insect two-hybrid assays to show that FTZ-F1 interacts with two JH receptor candidates, the bHLH-PAS paralogs MET and GCE, in a JH-dependent manner. These interactions are severely reduced when helix 12 of the FTZ-F1 activation function 2 (AF2) is removed, implicating AF2 as an interacting site. Through homology modeling, we found that MET and GCE possess a C-terminal α-helix featuring a conserved motif LIXXL that represents a novel nuclear receptor (NR) box. Docking simulations supported by two-hybrid experiments revealed that FTZ-F1·MET and FTZ-F1·GCE heterodimer formation involves a typical NR box-AF2 interaction but does not require the canonical charge clamp residues of FTZ-F1 and relies primarily on hydrophobic contacts, including a unique interaction with helix 4. Moreover, we identified paralog-specific features, including a secondary interaction site found only in MET. Our findings suggest that a novel NR box enables MET and GCE to interact JH-dependently with the AF2 of FTZ-F1.
Figures





Similar articles
-
The Drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway.J Biol Chem. 2011 Sep 23;286(38):33689-700. doi: 10.1074/jbc.M111.273458. Epub 2011 Aug 8. J Biol Chem. 2011. PMID: 21832074 Free PMC article.
-
Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire.J Biol Chem. 2019 Jan 11;294(2):410-423. doi: 10.1074/jbc.RA118.005992. Epub 2018 Nov 19. J Biol Chem. 2019. PMID: 30455350 Free PMC article.
-
Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor.PLoS Genet. 2015 Jul 10;11(7):e1005394. doi: 10.1371/journal.pgen.1005394. eCollection 2015 Jul. PLoS Genet. 2015. PMID: 26161662 Free PMC article.
-
The juvenile hormone signaling pathway in insect development.Annu Rev Entomol. 2013;58:181-204. doi: 10.1146/annurev-ento-120811-153700. Epub 2012 Sep 17. Annu Rev Entomol. 2013. PMID: 22994547 Review.
-
Juvenile hormone action: a 2007 perspective.J Insect Physiol. 2008 Jun;54(6):895-901. doi: 10.1016/j.jinsphys.2008.01.014. Epub 2008 Feb 16. J Insect Physiol. 2008. PMID: 18355835 Review.
Cited by
-
Mapping of the Sequences Directing Localization of the Drosophila Germ Cell-Expressed Protein (GCE).PLoS One. 2015 Jul 17;10(7):e0133307. doi: 10.1371/journal.pone.0133307. eCollection 2015. PLoS One. 2015. PMID: 26186223 Free PMC article.
-
Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins.Insects. 2012 Mar 22;3(1):324-38. doi: 10.3390/insects3010324. Insects. 2012. PMID: 26467963 Free PMC article. Review.
-
Krüppel-homologue 1 regulates the development of Tuta absoluta and its cascade regulation pattern in the juvenile hormone signalling pathway.Open Biol. 2023 May;13(5):220372. doi: 10.1098/rsob.220372. Epub 2023 May 31. Open Biol. 2023. PMID: 37253420 Free PMC article.
-
The intrinsically disordered region of GCE protein adopts a more fixed structure by interacting with the LBD of the nuclear receptor FTZ-F1.Cell Commun Signal. 2020 Nov 5;18(1):180. doi: 10.1186/s12964-020-00662-2. Cell Commun Signal. 2020. PMID: 33153474 Free PMC article.
-
MET is required for the maximal action of 20-hydroxyecdysone during Bombyx metamorphosis.PLoS One. 2012;7(12):e53256. doi: 10.1371/journal.pone.0053256. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300902 Free PMC article.
References
-
- Dubrovsky E. B. (2005) Hormonal cross-talk in insect development. Trends Endocrinol. Metab. 16, 6–11 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

