Sequential class switching is required for the generation of high affinity IgE antibodies
- PMID: 22249450
- PMCID: PMC3280879
- DOI: 10.1084/jem.20111941
Sequential class switching is required for the generation of high affinity IgE antibodies
Abstract
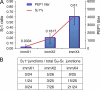
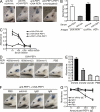
IgE antibodies with high affinity for their antigens can be stably cross-linked at low concentrations by trace amounts of antigen, whereas IgE antibodies with low affinity bind their antigens weakly. In this study, we find that there are two distinct pathways to generate high and low affinity IgE. High affinity IgE is generated through sequential class switching (μ→γ→ε) in which an intermediary IgG phase is necessary for the affinity maturation of the IgE response, where the IgE inherits somatic hypermutations and high affinity from the IgG1 phase. In contrast, low affinity IgE is generated through direct class switching (μ→ε) and is much less mutated. Mice deficient in IgG1 production cannot produce high affinity IgE, even after repeated immunizations. We demonstrate that a small amount of high affinity IgE can cause anaphylaxis and is pathogenic. Low affinity IgE competes with high affinity IgE for binding to Fcε receptors and prevents anaphylaxis and is thus beneficial.
Figures



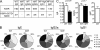

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

