Aberrant neuregulin 1 signaling in amyotrophic lateral sclerosis
- PMID: 22249457
- PMCID: PMC3270561
- DOI: 10.1097/NEN.0b013e3182423c43
Aberrant neuregulin 1 signaling in amyotrophic lateral sclerosis
Abstract
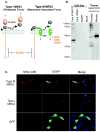
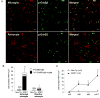
Neuregulin 1 (NRG1) is a neuron-derived trophic molecule that supports axoglial and neuromuscular development through several alternatively spliced isoforms; its possible role in the pathogenesis and progression of amyotrophic lateral sclerosis (ALS) is not known. We analyzed the relationship of NRG1 isoform expression to glial cell activation and motor neuron loss in spinal cords of ALS patients and during disease progression in the superoxide dismutase 1 (SOD1) ALS mouse model. Microgliosis, astrocytosis, and motor neuron loss were observed in the ventral horns in ALS patients and were increased in SOD1 mice along with disease progression. Type III (membrane-bound) NRG1 expression was reduced in parallel with motor neuron loss, but Type I (secreted) NRG1 expression was increased and was associated with glial activation. Increased NRG1 receptor activation was observed on activated microglia in both ALS patients and in SOD1 mice. This activation was observed at the time of disease onset and before upregulation of NRG1 gene expression in the mice. The downregulation of membrane-bound Type III NRG1 forms may reflect motor neuron loss, but increased signaling by secreted-type NRG1 isoforms could contribute to disease pathogenesis through glial cell activation. NRG1 might, therefore, represent a novel therapeutic target against disease progression in ALS.
Figures






Similar articles
-
Neuregulin 1 confers neuroprotection in SOD1-linked amyotrophic lateral sclerosis mice via restoration of C-boutons of spinal motor neurons.Acta Neuropathol Commun. 2016 Feb 18;4:15. doi: 10.1186/s40478-016-0286-7. Acta Neuropathol Commun. 2016. PMID: 26891847 Free PMC article.
-
Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis.J Neuroinflammation. 2010 Jan 28;7:8. doi: 10.1186/1742-2094-7-8. J Neuroinflammation. 2010. PMID: 20109233 Free PMC article.
-
Therapeutic Role of Neuregulin 1 Type III in SOD1-Linked Amyotrophic Lateral Sclerosis.Neurotherapeutics. 2020 Jul;17(3):1048-1060. doi: 10.1007/s13311-019-00811-7. Neurotherapeutics. 2020. PMID: 31965551 Free PMC article.
-
Activation of microglial neuregulin1 signaling in the corticospinal tracts of ALS patients with upper motor neuron signs.Amyotroph Lateral Scler Frontotemporal Degener. 2014 Mar;15(1-2):77-83. doi: 10.3109/21678421.2013.853802. Epub 2013 Nov 14. Amyotroph Lateral Scler Frontotemporal Degener. 2014. PMID: 24229388
-
Quantifying disease progression in amyotrophic lateral sclerosis.Ann Neurol. 2014 Nov;76(5):643-57. doi: 10.1002/ana.24273. Epub 2014 Sep 30. Ann Neurol. 2014. PMID: 25223628 Free PMC article. Review.
Cited by
-
Specific Expression of Glial-Derived Neurotrophic Factor in Muscles as Gene Therapy Strategy for Amyotrophic Lateral Sclerosis.Neurotherapeutics. 2021 Apr;18(2):1113-1126. doi: 10.1007/s13311-021-01025-6. Epub 2021 Mar 30. Neurotherapeutics. 2021. PMID: 33786805 Free PMC article.
-
TDP-43 proteinopathy in ALS is triggered by loss of ASRGL1 and associated with HML-2 expression.Nat Commun. 2024 May 16;15(1):4163. doi: 10.1038/s41467-024-48488-7. Nat Commun. 2024. PMID: 38755145 Free PMC article.
-
Epigenetic age acceleration is associated with occupational exposures, sex, and survival in amyotrophic lateral sclerosis.EBioMedicine. 2024 Nov;109:105383. doi: 10.1016/j.ebiom.2024.105383. Epub 2024 Oct 5. EBioMedicine. 2024. PMID: 39369616 Free PMC article.
-
Gene Therapy Overexpressing Neuregulin 1 Type I in Combination With Neuregulin 1 Type III Promotes Functional Improvement in the SOD1G93A ALS Mice.Front Neurol. 2021 Sep 22;12:693309. doi: 10.3389/fneur.2021.693309. eCollection 2021. Front Neurol. 2021. PMID: 34630277 Free PMC article.
-
Neuregulin 1 Reduces Motoneuron Cell Death and Promotes Neurite Growth in an in Vitro Model of Motoneuron Degeneration.Front Cell Neurosci. 2018 Jan 9;11:431. doi: 10.3389/fncel.2017.00431. eCollection 2017. Front Cell Neurosci. 2018. PMID: 29375317 Free PMC article.
References
-
- Cruz-Sanchez FF, Moral A, Rossi ML, et al. Synaptophysin in spinal anterior horn in aging and ALS: an immunohistological study. J Neural Transm. 1996;103:1317–29. - PubMed
-
- Ravits J, Laurie P, Fan Y, et al. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68:1576–82. - PubMed
-
- Walling AD. Amyotrophic lateral sclerosis: Lou Gehrig’s disease. Am Fam Physician. 1999;59:1489–96. - PubMed
-
- Gordon PH, Cheng B, Katz IB, et al. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology. 2009;72:1948–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

