Cerebellar ataxia in patients with mitochondrial DNA disease: a molecular clinicopathological study
- PMID: 22249460
- PMCID: PMC3272439
- DOI: 10.1097/NEN.0b013e318244477d
Cerebellar ataxia in patients with mitochondrial DNA disease: a molecular clinicopathological study
Abstract
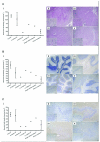
Cerebellar ataxia is a prominent clinical symptom in patients with mitochondrial DNA (mtDNA) disease. This is often progressive with onset in young adulthood. We performed a detailed neuropathologic investigation of the olivary-cerebellum in 14 genetically and clinically well-defined patients with mtDNA disease. Quantitative neuropathologic investigation showed varying levels of loss of Purkinje cells and neurons of the dentate nucleus and inferior olivary nuclei. Typically, focal Purkinje cell loss was present in patients with the m.3243A>G mutation caused by the presence of microinfarcts, with relative preservation of neuronal cell populations in the olivary and dentate nuclei. In contrast, patients with the m.8344A>G mutation or recessive POLG mutations showed extensive and global neuronal cell loss in all 3 olivary-cerebellum areas examined. Molecular analysis of mutated mtDNA heteroplasmy levels revealed that neuronal cell loss occurred independently of the level of mutated mtDNA present within surviving neurons. High levels of neuronal respiratory chain deficiency, particularly of complex I, were detected in surviving cells; levels of deficiency were greater in regions with extensive cell loss. We found a relationship between respiratory deficiency and neuronal cell density, indicating that neuronal cell death correlates with respiratory deficiency. These findings highlight the vulnerability of the olivary-cerebellum to mtDNA defects.
Figures



References
-
- McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9:829–40. - PubMed
-
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol. 2002;120:791–6. - PubMed
-
- Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0700718(84679)/MRC_/Medical Research Council/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0700718/MRC_/Medical Research Council/United Kingdom
- G0502157/MRC_/Medical Research Council/United Kingdom
- 096919/WT_/Wellcome Trust/United Kingdom
- 074454/Z/04/Z/WT_/Wellcome Trust/United Kingdom
- MC_G0802536/MRC_/Medical Research Council/United Kingdom
- 074454/WT_/Wellcome Trust/United Kingdom
- G1100540/MRC_/Medical Research Council/United Kingdom
- G0800674/MRC_/Medical Research Council/United Kingdom
- G0400074/MRC_/Medical Research Council/United Kingdom
- G0900652/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

