Reduced occurrence of programmed cell death and gliosis in the retinas of juvenile rabbits after shortterm treatment with intravitreous bevacizumab
- PMID: 22249482
- PMCID: PMC3248603
- DOI: 10.6061/clinics/2012(01)10
Reduced occurrence of programmed cell death and gliosis in the retinas of juvenile rabbits after shortterm treatment with intravitreous bevacizumab
Abstract
Objective: Bevacizumab has been widely used as a vascular endothelial growth factor antagonist in the treatment of retinal vasoproliferative disorders in adults and, more recently, in infants with retinopathy of prematurity. Recently, it has been proposed that vascular endothelial growth factor acts as a protective factor for neurons and glial cells, particularly in developing nervous tissue. The purpose of this study was to investigate the effects of bevacizumab on the developing retinas of juvenile rabbits.
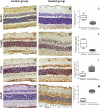
Methods: Juvenile rabbits received bevacizumab intravitreously in one eye; the other eye acted as an untreated control. Slit-lamp and fundoscopic examinations were performed both prior to and seven days after treatment. At the same time, retina samples were analyzed using immunohistochemistry to detect autophagy and apoptosis as well as proliferation and glial reactivity. Morphometric analyses were performed, and the data were analyzed using the Mann-Whitney U test.
Results: No clinical abnormalities were observed in either treated or untreated eyes. However, immunohistochemical analyses revealed a reduction in the occurrence of programmed cell death and increases in both proliferation and reactivity in the bevacizumab-treated group compared with the untreated group.
Conclusions: Bevacizumab appears to alter programmed cell death patterns and promote gliosis in the developing retinas of rabbits; therefore, it should be used with caution in developing eyes.
Conflict of interest statement
No potential conflict of interest was reported.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

