Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism
- PMID: 22249518
- PMCID: PMC4489419
- DOI: 10.1038/nrendo.2011.218
Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism
Abstract
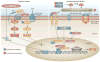
The discovery of fibroblast growth factor 23 (FGF-23) has expanded our understanding of phosphate and vitamin D homeostasis and provided new insights into the pathogenesis of hereditary hypophosphatemic and hyperphosphatemic disorders, as well as acquired disorders of phosphate metabolism, such as chronic kidney disease. FGF-23 is secreted by osteoblasts and osteocytes in bone and principally targets the kidney to regulate the reabsorption of phosphate, the production and catabolism of 1,25-dihydroxyvitamin D and the expression of α-Klotho, an anti-ageing hormone. Secreted FGF-23 plays a central role in complex endocrine networks involving local bone-derived factors that regulate mineralization of extracellular matrix and systemic hormones involved in mineral metabolism. Inactivating mutations of PHEX, DMP1 and ENPP1, which cause hereditary hypophosphatemic disorders and primary defects in bone mineralization, stimulate FGF23 gene transcription in osteoblasts and osteocytes, at least in part, through canonical and intracrine FGF receptor pathways. These FGF-23 regulatory pathways may enable systemic phosphate and vitamin D homeostasis to be coordinated with bone mineralization. FGF-23 also functions as a counter-regulatory hormone for 1,25-dihydroxyvitamin D in a bone-kidney endocrine loop. FGF-23, through regulation of additional genes in the kidney and extrarenal tissues, probably has broader physiological functions beyond regulation of mineral metabolism that account for the association between FGF-23 and increased mortality and morbidity in chronic kidney disease.
Conflict of interest statement
The author declares associations with the following companies: Amgen, KAI Pharmaceuticals. See the article online for full details of the relationships.
Figures
Similar articles
-
Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization.Curr Opin Nephrol Hypertens. 2007 Jul;16(4):329-35. doi: 10.1097/MNH.0b013e3281ca6ffd. Curr Opin Nephrol Hypertens. 2007. PMID: 17565275 Review.
-
Bone mineralization is regulated by signaling cross talk between molecular factors of local and systemic origin: the role of fibroblast growth factor 23.Biofactors. 2014 Nov-Dec;40(6):555-68. doi: 10.1002/biof.1186. Epub 2014 Oct 29. Biofactors. 2014. PMID: 25352227 Review.
-
Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease.Exp Cell Res. 2012 May 15;318(9):1040-8. doi: 10.1016/j.yexcr.2012.02.027. Epub 2012 Mar 7. Exp Cell Res. 2012. PMID: 22421513 Free PMC article. Review.
-
Multiple faces of fibroblast growth factor-23.Curr Opin Nephrol Hypertens. 2016 Jul;25(4):333-42. doi: 10.1097/MNH.0000000000000240. Curr Opin Nephrol Hypertens. 2016. PMID: 27219044 Free PMC article. Review.
-
Novel regulators of phosphate homeostasis and bone metabolism.Ther Apher Dial. 2007 Oct;11 Suppl 1:S3-22. doi: 10.1111/j.1744-9987.2007.00513.x. Ther Apher Dial. 2007. PMID: 17976082 Review.
Cited by
-
Osteocyte-derived insulin-like growth factor I is not essential for the bone repletion response in mice.PLoS One. 2015 Jan 30;10(1):e0115897. doi: 10.1371/journal.pone.0115897. eCollection 2015. PLoS One. 2015. PMID: 25635763 Free PMC article.
-
Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model.Sci Rep. 2015 Jun 9;5:11049. doi: 10.1038/srep11049. Sci Rep. 2015. PMID: 26056071 Free PMC article.
-
Congenital Conditions of Hypophosphatemia Expressed in Adults.Calcif Tissue Int. 2021 Jan;108(1):91-103. doi: 10.1007/s00223-020-00695-2. Epub 2020 May 14. Calcif Tissue Int. 2021. PMID: 32409880 Review.
-
The Osteocyte: New Insights.Annu Rev Physiol. 2020 Feb 10;82:485-506. doi: 10.1146/annurev-physiol-021119-034332. Annu Rev Physiol. 2020. PMID: 32040934 Free PMC article. Review.
-
A novel c.2179T>C mutation blocked the intracellular transport of PHEX protein and caused X-linked hypophosphatemic rickets in a Chinese family.Mol Genet Genomic Med. 2020 Aug;8(8):e1262. doi: 10.1002/mgg3.1262. Epub 2020 Jun 8. Mol Genet Genomic Med. 2020. PMID: 32511895 Free PMC article.
References
-
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

