The economic pressures for biosimilar drug use in cancer medicine
- PMID: 22249658
- PMCID: PMC3291824
- DOI: 10.1007/s11523-011-0196-3
The economic pressures for biosimilar drug use in cancer medicine
Abstract
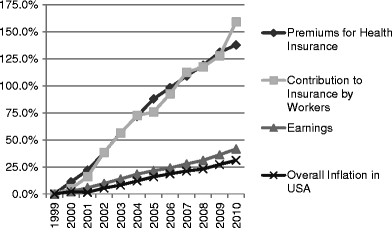
The main rationale for using biosimilar drugs is for cost saving. The market development for biosimilar drugs will therefore depend on the degree to which cost saving measures are required by nations, medical insurers and individuals and the absolute savings that could be gained by switching from original drugs. This paper is designed to discover the degree to which financial constraints will drive future health spending and to discover if legal or safety issues could impact on any trend. A structured literature search was performed for papers and documents to 27 August 2011. Where multiple sources of data were available on a topic, data from papers and reports by multinational or national bodies were used in preference to data from regions or individual hospitals. Almost all health systems face current significant cost pressures. The twin driver of increasing cancer prevalence as populations age and cancer medicine costs rising faster than inflation places oncology as the most significant single cost problem. For some countries, this is predicted to make medicine unaffordable within a decade. Most developed countries have planned to embrace biosimilar use as a cost-control measure. Biosimilar introduction into the EU has already forced prices down, both the price of biosimilar drugs and competitive price reductions in originator drugs. Compound annual growth rates of use have been predicted at 65.8% per year. Most developed countries have planned to embrace biosimilar use as a major cost-control measure. Only legal blocks and safety concerns are likely to act against this trend. For centralised healthcare systems, and those with a strong tradition of generic medicine use, biosimilar use will clearly rise with predictions of more than 80% of prescriptions of some biologic drugs within 1 year of market entry in the USA. Delaying the implementation of such programmes however risks a real crisis in healthcare delivery for many countries and hospitals that few can now afford.
Figures



References
-
- Mulcahy N (2009) Time to consider cost in evaluating cancer drugs in United States? Medscape Medical News July 14. http://www.medscape.com/viewarticle/705689
-
- Behnke N, Hueltenschmidt N, Pasternak A, Singh K (2011) Biosimilars: a marathon, not a sprint. Industry Brief 12/16/09. http://www.bain.com/bainweb/publications/publications_detail.asp?id=2751...
-
- Medicare Payment Advisory Commission: report to the Congress: Medicare Payment Policy, (3, 2010) (Washington, DC). Available at http://www.medpac.gov/documents/Mar10_EntireReport.pdf. Accessed 3 March 2011
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

