Characterization of KCNQ1 atrial fibrillation mutations reveals distinct dependence on KCNE1
- PMID: 22250012
- PMCID: PMC3269792
- DOI: 10.1085/jgp.201110672
Characterization of KCNQ1 atrial fibrillation mutations reveals distinct dependence on KCNE1
Abstract
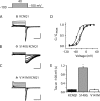
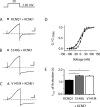
The I(Ks) potassium channel, critical to control of heart electrical activity, requires assembly of α (KCNQ1) and β (KCNE1) subunits. Inherited mutations in either I(Ks) channel subunit are associated with cardiac arrhythmia syndromes. Two mutations (S140G and V141M) that cause familial atrial fibrillation (AF) are located on adjacent residues in the first membrane-spanning domain of KCNQ1, S1. These mutations impair the deactivation process, causing channels to appear constitutively open. Previous studies suggest that both mutant phenotypes require the presence of KCNE1. Here we found that despite the proximity of these two mutations in the primary protein structure, they display different functional dependence in the presence of KCNE1. In the absence of KCNE1, the S140G mutation, but not V141M, confers a pronounced slowing of channel deactivation and a hyperpolarizing shift in voltage-dependent activation. When coexpressed with KCNE1, both mutants deactivate significantly slower than wild-type KCNQ1/KCNE1 channels. The differential dependence on KCNE1 can be correlated with the physical proximity between these positions and KCNE1 as shown by disulfide cross-linking studies: V141C forms disulfide bonds with cysteine-substituted KCNE1 residues, whereas S140C does not. These results further our understanding of the structural relationship between KCNE1 and KCNQ1 subunits in the I(Ks) channel, and provide mechanisms for understanding the effects on channel deactivation underlying these two atrial fibrillation mutations.
Figures





Similar articles
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
-
Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels.J Physiol. 2008 Sep 1;586(17):4179-91. doi: 10.1113/jphysiol.2008.157511. Epub 2008 Jul 3. J Physiol. 2008. PMID: 18599533 Free PMC article.
-
Selective targeting of gain-of-function KCNQ1 mutations predisposing to atrial fibrillation.Circ Arrhythm Electrophysiol. 2013 Oct;6(5):960-6. doi: 10.1161/CIRCEP.113.000439. Epub 2013 Sep 4. Circ Arrhythm Electrophysiol. 2013. PMID: 24006450 Free PMC article.
-
Gating mechanisms underlying deactivation slowing by two KCNQ1 atrial fibrillation mutations.Sci Rep. 2017 Apr 6;7:45911. doi: 10.1038/srep45911. Sci Rep. 2017. PMID: 28383569 Free PMC article.
-
Recent molecular insights from mutated IKS channels in cardiac arrhythmia.Curr Opin Pharmacol. 2014 Apr;15:74-82. doi: 10.1016/j.coph.2013.12.004. Epub 2013 Dec 30. Curr Opin Pharmacol. 2014. PMID: 24721657 Review.
Cited by
-
KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain.J Physiol. 2015 Jun 15;593(12):2617-25. doi: 10.1113/jphysiol.2014.287672. Epub 2015 Feb 16. J Physiol. 2015. PMID: 25603957 Free PMC article. Review.
-
ML277 specifically enhances the fully activated open state of KCNQ1 by modulating VSD-pore coupling.Elife. 2019 Jul 22;8:e48576. doi: 10.7554/eLife.48576. Elife. 2019. PMID: 31329101 Free PMC article.
-
Probing the structural basis for differential KCNQ1 modulation by KCNE1 and KCNE2.J Gen Physiol. 2012 Dec;140(6):653-69. doi: 10.1085/jgp.201210847. J Gen Physiol. 2012. PMID: 23183700 Free PMC article.
-
Structures Illuminate Cardiac Ion Channel Functions in Health and in Long QT Syndrome.Front Pharmacol. 2020 May 4;11:550. doi: 10.3389/fphar.2020.00550. eCollection 2020. Front Pharmacol. 2020. PMID: 32431610 Free PMC article. Review.
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

