Activation of the NLRP3 inflammasome by group B streptococci
- PMID: 22250086
- PMCID: PMC3273589
- DOI: 10.4049/jimmunol.1102543
Activation of the NLRP3 inflammasome by group B streptococci
Abstract
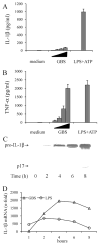
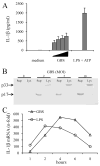
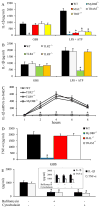
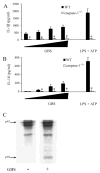
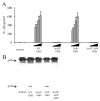
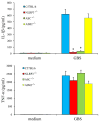
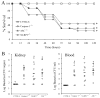
Group B Streptococcus (GBS) is a frequent agent of life-threatening sepsis and meningitis in neonates and adults with predisposing conditions. We tested the hypothesis that activation of the inflammasome, an inflammatory signaling complex, is involved in host defenses against this pathogen. We show in this study that murine bone marrow-derived conventional dendritic cells responded to GBS by secreting IL-1β and IL-18. IL-1β release required both pro-IL-1β transcription and caspase-1-dependent proteolytic cleavage of intracellular pro-IL-1β. Dendritic cells lacking the TLR adaptor MyD88, but not those lacking TLR2, were unable to produce pro-IL-1β mRNA in response to GBS. Pro-IL-1β cleavage and secretion of the mature IL-1β form depended on the NOD-like receptor family, pyrin domain containing 3 (NLRP3) sensor and the apoptosis-associated speck-like protein containing a caspase activation and recruitment domain adaptor. Moreover, activation of the NLRP3 inflammasome required GBS expression of β-hemolysin, an important virulence factor. We further found that mice lacking NLRP3, apoptosis-associated speck-like protein, or caspase-1 were considerably more susceptible to infection than wild-type mice. Our data link the production of a major virulence factor by GBS with the activation of a highly effective anti-GBS response triggered by the NLRP3 inflammasome.
Conflict of interest statement
The authors have no financial conflicts of interest
Figures
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. - PubMed
-
- Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20:3–9. - PubMed
-
- Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Núñez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. - PubMed
-
- Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

