Eosinophils in glioblastoma biology
- PMID: 22251275
- PMCID: PMC3269388
- DOI: 10.1186/1742-2094-9-11
Eosinophils in glioblastoma biology
Abstract
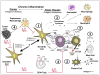
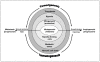
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults. The development of this malignant glial lesion involves a multi-faceted process that results in a loss of genetic or epigenetic gene control, un-regulated cell growth, and immune tolerance. Of interest, atopic diseases are characterized by a lack of immune tolerance and are inversely associated with glioma risk. One cell type that is an established effector cell in the pathobiology of atopic disease is the eosinophil. In response to various stimuli, the eosinophil is able to produce cytotoxic granules, neuromediators, and pro-inflammatory cytokines as well as pro-fibrotic and angiogenic factors involved in pathogen clearance and tissue remodeling and repair. These various biological properties reveal that the eosinophil is a key immunoregulatory cell capable of influencing the activity of both innate and adaptive immune responses. Of central importance to this report is the observation that eosinophil migration to the brain occurs in response to traumatic brain injury and following certain immunotherapeutic treatments for GBM. Although eosinophils have been identified in various central nervous system pathologies, and are known to operate in wound/repair and tumorstatic models, the potential roles of eosinophils in GBM development and the tumor immunological response are only beginning to be recognized and are therefore the subject of the present review.
Figures


Similar articles
-
Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells.Int J Cancer. 2015 Jun 1;136(11):2566-78. doi: 10.1002/ijc.29309. Epub 2014 Nov 12. Int J Cancer. 2015. PMID: 25363661
-
Eosinophils and other peripheral blood biomarkers in glioma grading: a preliminary study.BMC Neurol. 2019 Dec 5;19(1):313. doi: 10.1186/s12883-019-1549-2. BMC Neurol. 2019. PMID: 31805879 Free PMC article.
-
Understanding the role of cytokines in Glioblastoma Multiforme pathogenesis.Cancer Lett. 2012 Mar 28;316(2):139-50. doi: 10.1016/j.canlet.2011.11.001. Epub 2011 Nov 7. Cancer Lett. 2012. PMID: 22075379 Review.
-
Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme.J Exp Clin Cancer Res. 2018 Oct 30;37(1):265. doi: 10.1186/s13046-018-0941-x. J Exp Clin Cancer Res. 2018. PMID: 30376874 Free PMC article.
-
Human cytomegalovirus-mediated immunomodulation: Effects on glioblastoma progression.Biochim Biophys Acta Rev Cancer. 2017 Aug;1868(1):273-276. doi: 10.1016/j.bbcan.2017.05.006. Epub 2017 May 26. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28554666 Review.
Cited by
-
Neuroinflammation: A Signature or a Cause of Epilepsy?Int J Mol Sci. 2021 Jun 29;22(13):6981. doi: 10.3390/ijms22136981. Int J Mol Sci. 2021. PMID: 34209535 Free PMC article. Review.
-
IL-4 in the brain: a cytokine to remember.J Immunol. 2012 Nov 1;189(9):4213-9. doi: 10.4049/jimmunol.1202246. J Immunol. 2012. PMID: 23087426 Free PMC article. Review.
-
Traumatic brain injury and subsequent glioblastoma development: Review of the literature and case reports.Surg Neurol Int. 2016 Aug 26;7:78. doi: 10.4103/2152-7806.189296. eCollection 2016. Surg Neurol Int. 2016. PMID: 27625888 Free PMC article.
-
Interleukin-33 in human gliomas: Expression and prognostic significance.Oncol Lett. 2016 Jul;12(1):445-452. doi: 10.3892/ol.2016.4626. Epub 2016 May 25. Oncol Lett. 2016. PMID: 27347163 Free PMC article.
-
DNA methylation heterogeneity attributable to a complex tumor immune microenvironment prompts prognostic risk in glioma.Epigenetics. 2024 Dec;19(1):2318506. doi: 10.1080/15592294.2024.2318506. Epub 2024 Mar 5. Epigenetics. 2024. PMID: 38439715 Free PMC article.
References
-
- Ito N, Hasegawa R, Imaida K, Hirose M, Asamoto M, Shirai T. Concepts in multistage carcinogenesis. Crit Rev Oncol Hematol. 1995;21(1-3):105–133. - PubMed
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. - PubMed
-
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

