Gap1 functions as a molecular chaperone to stabilize its interactive partner Gap3 during biogenesis of serine-rich repeat bacterial adhesin
- PMID: 22251284
- PMCID: PMC3285465
- DOI: 10.1111/j.1365-2958.2012.07970.x
Gap1 functions as a molecular chaperone to stabilize its interactive partner Gap3 during biogenesis of serine-rich repeat bacterial adhesin
Abstract
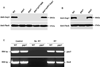
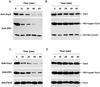
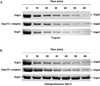
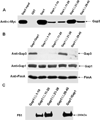
Serine-rich repeat glycoproteins (SRRPs) are important bacterial adhesins that are conserved in streptococci and staphylococci. Fimbriae-associated protein (Fap1) from Streptococcus parasanguinis, was the first SRRP identified; it plays an important role in bacterial biofilm formation. A gene cluster encoding glycosyltransferases and accessory secretion components is required for Fap1 biogenesis. Two glycosylation-associated proteins, Gap1 and Gap3 within the cluster, interact with each other and function in concert in Fap1 biogenesis. Here we report the new molecular events underlying contribution of the interaction to Fap1 biogenesis. The Gap1-deficient mutant rendered Gap3 unstable and degraded in vitro and in vivo. Inactivation of a gene encoding protease ClpP reversed the phenotype of the gap1 mutant, suggesting that ClpP is responsible for degradation of Gap3. Molecular chaperone GroEL was co-purified with Gap3 only when Gap1 was absent and also reacted with Gap1 monoclonal antibody, suggesting that Gap1 functions as a specific chaperone for Gap3. The N-terminal interacting domains of Gap1 mediated the Gap3 stability and Fap1 biogenesis. Gap1 homologues from Streptococcus agalactiae and Staphylococcus aureus also interacted with and stabilized corresponding Gap3 homologues, suggesting that the chaperone activity of the Gap1 homologues is common in biogenesis of SRRPs.
© 2012 Blackwell Publishing Ltd.
Figures



Similar articles
-
A conserved domain of previously unknown function in Gap1 mediates protein-protein interaction and is required for biogenesis of a serine-rich streptococcal adhesin.Mol Microbiol. 2008 Dec;70(5):1094-104. doi: 10.1111/j.1365-2958.2008.06456.x. Epub 2008 Sep 30. Mol Microbiol. 2008. PMID: 18826412 Free PMC article.
-
Canonical SecA associates with an accessory secretory protein complex involved in biogenesis of a streptococcal serine-rich repeat glycoprotein.J Bacteriol. 2011 Dec;193(23):6560-6. doi: 10.1128/JB.05668-11. Epub 2011 Sep 30. J Bacteriol. 2011. PMID: 21965576 Free PMC article.
-
Gap2 promotes the formation of a stable protein complex required for mature Fap1 biogenesis.J Bacteriol. 2013 May;195(10):2166-76. doi: 10.1128/JB.02255-12. Epub 2013 Mar 8. J Bacteriol. 2013. PMID: 23475979 Free PMC article.
-
Glycosylation and biogenesis of a family of serine-rich bacterial adhesins.Microbiology (Reading). 2009 Feb;155(Pt 2):317-327. doi: 10.1099/mic.0.025221-0. Microbiology (Reading). 2009. PMID: 19202081 Review.
-
Glycosyltransferase-mediated Sweet Modification in Oral Streptococci.J Dent Res. 2015 May;94(5):659-65. doi: 10.1177/0022034515574865. Epub 2015 Mar 9. J Dent Res. 2015. PMID: 25755271 Free PMC article. Review.
Cited by
-
Modification of Streptococcus mutans Cnm by PgfS contributes to adhesion, endothelial cell invasion, and virulence.J Bacteriol. 2014 Aug;196(15):2789-97. doi: 10.1128/JB.01783-14. Epub 2014 May 16. J Bacteriol. 2014. PMID: 24837294 Free PMC article.
-
New Helical Binding Domain Mediates a Glycosyltransferase Activity of a Bifunctional Protein.J Biol Chem. 2016 Oct 14;291(42):22106-22117. doi: 10.1074/jbc.M116.731695. Epub 2016 Aug 17. J Biol Chem. 2016. PMID: 27539847 Free PMC article.
-
Selective transport by SecA2: an expanding family of customized motor proteins.Biochim Biophys Acta. 2014 Aug;1843(8):1674-86. doi: 10.1016/j.bbamcr.2013.10.019. Epub 2013 Oct 31. Biochim Biophys Acta. 2014. PMID: 24184206 Free PMC article. Review.
-
Fine-tuned production of hydrogen peroxide promotes biofilm formation of Streptococcus parasanguinis by a pathogenic cohabitant Aggregatibacter actinomycetemcomitans.Environ Microbiol. 2016 Nov;18(11):4023-4036. doi: 10.1111/1462-2920.13425. Epub 2016 Jul 22. Environ Microbiol. 2016. PMID: 27348605 Free PMC article.
-
Unraveling the sequence of cytosolic reactions in the export of GspB adhesin from Streptococcus gordonii.J Biol Chem. 2018 Apr 6;293(14):5360-5373. doi: 10.1074/jbc.RA117.000963. Epub 2018 Feb 9. J Biol Chem. 2018. PMID: 29462788 Free PMC article.
References
-
- Aldridge P, Karlinsey J, Hughes KT. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol Microbiol. 2003;49:1333–1345. - PubMed
-
- Arnesen T, Starheim KK, Van Damme P, Evjenth R, Dinh H, Betts MJ, Ryningen A, Vandekerckhove J, Gevaert K, Anderson D. The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation. Mol Cell Biol. 2010;30:1898–1909. - PMC - PubMed
-
- Bensing BA, Sullam PM. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol Microbiol. 2002;44:1081–1094. - PubMed
-
- Betiku E. Molecular Chaperones involved in Heterologous Protein Folding in Escherichia coli. Biotechnology and Molecular Biology Review. 2006;1:66–75.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

