Role of α-synuclein penetration into the membrane in the mechanisms of oligomer pore formation
- PMID: 22251432
- PMCID: PMC3925782
- DOI: 10.1111/j.1742-4658.2012.08489.x
Role of α-synuclein penetration into the membrane in the mechanisms of oligomer pore formation
Abstract
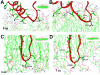
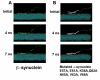
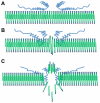
Parkinson's disease (PD) and dementia with Lewy bodies are common disorders of the aging population and characterized by the progressive accumulation of α-synuclein (α-syn) in the central nervous system. Aggregation of α-syn into oligomers with a ring-like appearance has been proposed to play a role in toxicity. However, the molecular mechanisms and the potential sequence of events involved in the formation of pore-like structures are unclear. We utilized computer modeling and cell-based studies to investigate the process of oligomerization of wild-type and A53T mutant α-syn in membranes. The studies suggest that α-syn penetrates the membrane rapidly, changing its conformation from α-helical towards a coiled structure. This penetration facilitates the incorporation of additional α-syn monomers in the complex, and the subsequent displacement of phospholipids and the formation of oligomers in the membrane. This process occurred more rapidly, and with a more favorable energy of interaction, for mutant A53T compared with wild-type α-syn. After 4 ns of simulation of the protein-membrane model, α-syn had penetrated through two-thirds of the membrane. By 9 ns, the penetration of the annular α-syn oligomers can result in the formation of pore-like structures that fully perforate the lipid bilayer. Experimental incubation of recombinant α-syn in synthetic membranes resulted in the formation of similar pore-like complexes. Moreover, mutant (A53T) α-syn had a greater tendency to accumulate in neuronal membrane fractions in cell cultures, resulting in greater neuronal permeability, as demonstrated with the calcein efflux assay. These studies provide a sequential molecular explanation for the process of α-syn oligomerization in the membrane, and support the role of formation of pore-like structures in the pathogenesis of the neurodegenerative process in PD.
© 2012 The Authors Journal compilation © 2012 FEBS.
Figures






References
-
- Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. - PubMed
-
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
-
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

