Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial
- PMID: 22251436
- PMCID: PMC3392799
- DOI: 10.1186/ar3685
Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial
Abstract
Introduction: The purpose of this study was to determine whether maraviroc, a human CC chemokine receptor 5 (CCR5) antagonist, is safe and effective in the treatment of active rheumatoid arthritis (RA) in patients on background methotrexate (MTX).
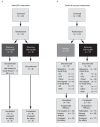
Methods: This phase IIa study comprised two distinct components: an open-label safety study of the pharmacokinetics (PK) of MTX in the presence of maraviroc, and a randomized, double-blind, placebo-controlled, proof-of-concept (POC) component. In the PK component, patients were randomized 1:1 to receive maraviroc 150 or 300 mg twice daily (BID) for four weeks. In the POC component, patients were randomized 2:1 to receive maraviroc 300 mg BID or placebo for 12 weeks. Patients were not eligible for inclusion in both components.
Results: Sixteen patients were treated in the safety/PK component. Maraviroc was well tolerated and there was no evidence of drug-drug interaction with MTX. One hundred ten patients were treated in the POC component. The study was terminated after the planned interim futility analysis due to lack of efficacy, at which time 59 patients (38 maraviroc; 21 placebo) had completed their week 12 visit. There was no significant difference in the number of ACR20 responders between the maraviroc (23.7%) and placebo (23.8%) groups (treatment difference -0.13%; 90% CI -20.45, 17.70; P = 0.504). The most common all-causality treatment-emergent adverse events in the maraviroc group were constipation (7.8%), nausea (5.2%), and fatigue (3.9%).
Conclusions: Maraviroc was generally well tolerated over 12 weeks; however, selective antagonism of CCR5 with maraviroc 300 mg BID failed to improve signs and symptoms in patients with active RA on background MTX.
Trial registration: ClinicalTrials.gov: NCT00427934.
Figures
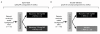

Comment in
-
What is the future of CCR5 antagonists in rheumatoid arthritis?Arthritis Res Ther. 2012 Mar 30;14(2):114. doi: 10.1186/ar3775. Arthritis Res Ther. 2012. PMID: 22494450 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

