Sigma-2 ligands induce tumour cell death by multiple signalling pathways
- PMID: 22251921
- PMCID: PMC3322954
- DOI: 10.1038/bjc.2011.602
Sigma-2 ligands induce tumour cell death by multiple signalling pathways
Abstract
Background: The sigma-2 receptor has been identified as a biomarker of proliferating cells in solid tumours. In the present study, we studied the mechanisms of sigma-2 ligand-induced cell death in the mouse breast cancer cell line EMT-6 and the human melanoma cell line MDA-MB-435.
Methods: EMT-6 and MDA-MB-435 cells were treated with sigma-2 ligands. The modulation of multiple signaling pathways of cell death was evaluated.
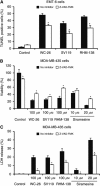
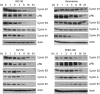
Results: Three sigma-2 ligands (WC-26, SV119 and RHM-138) induced DNA fragmentation, caspase-3 activation and PARP-1 cleavage. The caspase inhibitor Z-VAD-FMK partially blocked DNA fragmentation and cytotoxicity caused by these compounds. These data suggest that sigma-2 ligand-induced apoptosis and caspase activation are partially responsible for the cell death. WC-26 and siramesine induced formation of vacuoles in the cells. WC-26, SV119, RHM-138 and siramesine increased the synthesis and processing of microtubule-associated protein light chain 3, an autophagosome marker, and decreased the expression levels of the downstream effectors of mammalian target of rapamycin (mTOR), p70S6K and 4EBP1, suggesting that sigma-2 ligands induce autophagy, probably by inhibition of the mTOR pathway. All four sigma-2 ligands decreased the expression of cyclin D1 in a time-dependent manner. In addition, WC-26 and SV119 mainly decreased cyclin B1, E2 and phosphorylation of retinoblastoma protein (pRb); RHM-138 mainly decreased cyclin E2; and 10 μM siramesine mainly decreased cyclin B1 and pRb. These data suggest that sigma-2 ligands also impair cell-cycle progression in multiple phases of the cell cycle.
Conclusion: Sigma-2 ligands induce cell death by multiple signalling pathways.
Figures

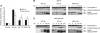


Similar articles
-
Sigma-2 ligands and PARP inhibitors synergistically trigger cell death in breast cancer cells.Biochem Biophys Res Commun. 2017 May 6;486(3):788-795. doi: 10.1016/j.bbrc.2017.03.122. Epub 2017 Mar 24. Biochem Biophys Res Commun. 2017. PMID: 28347815
-
Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer.J Exp Clin Cancer Res. 2012 May 2;31(1):41. doi: 10.1186/1756-9966-31-41. J Exp Clin Cancer Res. 2012. PMID: 22551149 Free PMC article.
-
Involvement of apoptosis and autophagy in the death of RPMI 8226 multiple myeloma cells by two enantiomeric sigma receptor ligands.Bioorg Med Chem. 2014 Jan 1;22(1):221-33. doi: 10.1016/j.bmc.2013.11.033. Epub 2013 Nov 25. Bioorg Med Chem. 2014. PMID: 24331758
-
Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy.Med Res Rev. 2014 May;34(3):532-66. doi: 10.1002/med.21297. Epub 2013 Aug 6. Med Res Rev. 2014. PMID: 23922215 Review.
-
Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands.Curr Pharm Des. 2010;16(31):3519-37. doi: 10.2174/138161210793563365. Curr Pharm Des. 2010. PMID: 21050178 Review.
Cited by
-
Small-Molecule Modulators of Sigma1 and Sigma2/TMEM97 in the Context of Cancer: Foundational Concepts and Emerging Themes.Front Pharmacol. 2019 Oct 21;10:1141. doi: 10.3389/fphar.2019.01141. eCollection 2019. Front Pharmacol. 2019. PMID: 31695608 Free PMC article. Review.
-
Identification and characterization of MAM03055A: A novel bivalent sigma-2 receptor/TMEM97 ligand with cytotoxic activity.Eur J Pharmacol. 2021 Sep 5;906:174263. doi: 10.1016/j.ejphar.2021.174263. Epub 2021 Jun 16. Eur J Pharmacol. 2021. PMID: 34144027 Free PMC article.
-
Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity.Molecules. 2017 Aug 25;22(9):1408. doi: 10.3390/molecules22091408. Molecules. 2017. PMID: 28841173 Free PMC article. Review.
-
Characterization of CM572, a Selective Irreversible Partial Agonist of the Sigma-2 Receptor with Antitumor Activity.J Pharmacol Exp Ther. 2015 Aug;354(2):203-12. doi: 10.1124/jpet.115.224105. Epub 2015 Jun 1. J Pharmacol Exp Ther. 2015. PMID: 26034081 Free PMC article.
-
Propellanes as Rigid Scaffolds for the Stereodefined Attachment of σ-Pharmacophoric Structural Elements to Achieve σ Affinity.Int J Mol Sci. 2021 May 26;22(11):5685. doi: 10.3390/ijms22115685. Int J Mol Sci. 2021. PMID: 34073622 Free PMC article.
References
-
- Allan LA, Clarke PR (2007) Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26: 301–310 - PubMed
-
- Allan LA, Clarke PR (2008) A mechanism coupling cell division and the control of apoptosis. SEB Exp Biol Ser 59: 257–265 - PubMed
-
- Barbieri F, Sparatore A, Alama A, Novelli F, Bruzzo C, Sparatore F (2003) Novel sigma binding site ligands as inhibitors of cell proliferation in breast cancer. Oncol Res 13: 455–461 - PubMed
-
- Bem WT, Thomas GE, Mamone JY, Homan SM, Levy BK, Johnson FE, Coscia CJ (1991) Overexpression of sigma receptors in nonneural human tumors. Cancer Res 51: 6558–6562 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

