Small surfactant-like peptides can drive soluble proteins into active aggregates
- PMID: 22251949
- PMCID: PMC3398302
- DOI: 10.1186/1475-2859-11-10
Small surfactant-like peptides can drive soluble proteins into active aggregates
Abstract
Background: Inactive protein inclusion bodies occur commonly in Escherichia coli (E. coli) cells expressing heterologous proteins. Previously several independent groups have found that active protein aggregates or pseudo inclusion bodies can be induced by a fusion partner such as a cellulose binding domain from Clostridium cellulovorans (CBDclos) when expressed in E. coli. More recently we further showed that a short amphipathic helical octadecapeptide 18A (EWLKAFYEKVLEKLKELF) and a short beta structure peptide ELK16 (LELELKLKLELELKLK) have a similar property.
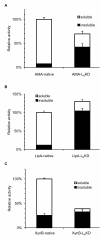
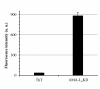
Results: In this work, we explored a third type of peptides, surfactant-like peptides, for performing such a "pulling-down" function. One or more of three such peptides (L6KD, L6K2, DKL6) were fused to the carboxyl termini of model proteins including Aspergillus fumigatus amadoriase II (AMA, all three peptides were used), Bacillus subtilis lipase A (LipA, only L6KD was used, hereinafter the same), Bacillus pumilus xylosidase (XynB), and green fluorescent protein (GFP), and expressed in E. coli. All fusions were found to predominantly accumulate in the insoluble fractions, with specific activities ranging from 25% to 92% of the native counterparts. Transmission electron microscopic (TEM) and confocal fluorescence microscopic analyses confirmed the formation of protein aggregates in the cell. Furthermore, binding assays with amyloid-specific dyes (thioflavin T and Cong red) to the AMA-L6KD aggregate and the TEM analysis of the aggregate following digestion with protease K suggested that the AMA-L6KD aggregate may contain structures reminiscent of amyloids, including a fibril-like structure core.
Conclusions: This study shows that the surfactant-like peptides L6KD and it derivatives can act as a pull-down handler for converting soluble proteins into active aggregates, much like 18A and ELK16. These peptide-mediated protein aggregations might have important implications for protein aggregation in vivo, and can be explored for production of functional biopolymers with detergent or other interfacial activities.
Figures



Similar articles
-
Active tyrosine phenol-lyase aggregates induced by terminally attached functional peptides in Escherichia coli.J Ind Microbiol Biotechnol. 2020 Aug;47(8):563-571. doi: 10.1007/s10295-020-02294-4. Epub 2020 Jul 31. J Ind Microbiol Biotechnol. 2020. PMID: 32737623 Free PMC article.
-
Formation of active inclusion bodies induced by hydrophobic self-assembling peptide GFIL8.Microb Cell Fact. 2015 Jun 16;14:88. doi: 10.1186/s12934-015-0270-0. Microb Cell Fact. 2015. PMID: 26077447 Free PMC article.
-
Streamlined protein expression and purification using cleavable self-aggregating tags.Microb Cell Fact. 2011 Jun 2;10:42. doi: 10.1186/1475-2859-10-42. Microb Cell Fact. 2011. PMID: 21631955 Free PMC article.
-
Active protein aggregates induced by terminally attached self-assembling peptide ELK16 in Escherichia coli.Microb Cell Fact. 2011 Feb 15;10:9. doi: 10.1186/1475-2859-10-9. Microb Cell Fact. 2011. PMID: 21320350 Free PMC article.
-
Cleavable self-aggregating tags (cSAT) for protein expression and purification.Methods Mol Biol. 2015;1258:65-78. doi: 10.1007/978-1-4939-2205-5_4. Methods Mol Biol. 2015. PMID: 25447859 Review.
Cited by
-
Why and how protein aggregation has to be studied in vivo.Microb Cell Fact. 2013 Feb 15;12:17. doi: 10.1186/1475-2859-12-17. Microb Cell Fact. 2013. PMID: 23410248 Free PMC article.
-
Screening and identification of genetic loci involved in producing more/denser inclusion bodies in Escherichia coli.Microb Cell Fact. 2013 May 2;12:43. doi: 10.1186/1475-2859-12-43. Microb Cell Fact. 2013. PMID: 23638724 Free PMC article.
-
Mutagenesis-Based Characterization and Improvement of a Novel Inclusion Body Tag.Front Bioeng Biotechnol. 2020 Jan 10;7:442. doi: 10.3389/fbioe.2019.00442. eCollection 2019. Front Bioeng Biotechnol. 2020. PMID: 31998707 Free PMC article.
-
Directed evolution of a genetically encoded immobilized lipase for the efficient production of biodiesel from waste cooking oil.Biotechnol Biofuels. 2019 Jun 28;12:165. doi: 10.1186/s13068-019-1509-5. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31297153 Free PMC article.
-
Active tyrosine phenol-lyase aggregates induced by terminally attached functional peptides in Escherichia coli.J Ind Microbiol Biotechnol. 2020 Aug;47(8):563-571. doi: 10.1007/s10295-020-02294-4. Epub 2020 Jul 31. J Ind Microbiol Biotechnol. 2020. PMID: 32737623 Free PMC article.
References
-
- Palmer I, Wingfield PT. In: Curr Protoc Protein Sci. Coligan JE, editor. Vol. 6. Hoboken: John Wiley & Sons Inc; 2004. Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli; pp. 6.3.1–6.3.15.
-
- Worrall DM, Goss NH. The formation of biologically active beta-galactosidase inclusion bodies in Escherichia coli. Aust J Biotechnol. 1989;3(1):28–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

