Recruiting adaptive cellular stress responses for successful brain ageing
- PMID: 22251954
- PMCID: PMC4084510
- DOI: 10.1038/nrn3151
Recruiting adaptive cellular stress responses for successful brain ageing
Abstract
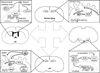
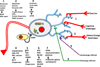
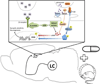
Successful ageing is determined in part by genetic background, but also by experiential factors associated with lifestyle and culture. Dietary, behavioural and pharmacological interventions have been identified as potential means to slow brain ageing and forestall neurodegenerative disease. Many of these interventions recruit adaptive cellular stress responses to strengthen neuronal networks and enhance plasticity. In this Science and Society article, we describe several determinants of healthy and pathological brain ageing, with insights into how these processes are accelerated or prevented. We also describe the mechanisms underlying the neuroprotective actions of exercise and nutritional interventions, with the goal of recruiting these molecular targets for the treatment and prevention of neurodegenerative disease.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

