The rationale for quadrivalent influenza vaccines
- PMID: 22252006
- PMCID: PMC3350141
- DOI: 10.4161/hv.8.1.17623
The rationale for quadrivalent influenza vaccines
Abstract
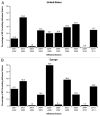
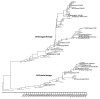
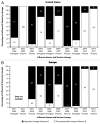
Two antigenically distinct lineages of influenza B viruses have circulated globally since 1985. However, licensed trivalent seasonal influenza vaccines contain antigens from only a single influenza B virus and thus provide limited immunity against circulating influenza B strains of the lineage not present in the vaccine. In recent years, predictions about which B lineage will predominate in an upcoming influenza season have been no better than chance alone, correct in only 5 of the 10 seasons from 2001 to 2011. Consequently, seasonal influenza vaccines could be improved by inclusion of influenza B strains of both lineages. The resulting quadrivalent influenza vaccines would allow influenza vaccination campaigns to respond more effectively to current global influenza epidemiology. Manufacturing capacity for seasonal influenza vaccines has increased sufficiently to supply quadrivalent influenza vaccines, and methods to identify the influenza B strains to include in such vaccines are in place. Multiple manufacturers have initiated clinical studies of quadrivalent influenza vaccines. Data from those studies, taken together with epidemiologic data regarding the burden of disease caused by influenza B infections, will determine the safety, effectiveness, and benefit of utilizing quadrivalent vaccines for the prevention of seasonal influenza disease.
Figures

References
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. - PubMed
-
- Couch RB. Background and presentation of possible vaccine options. Presented at: Food and Drug Administration, Center for Biologics Evaluation and Research, Vaccines and Related Biological Products Advisory Committee Meeting; February 27-28, 2007; Washington, DC.
-
- US Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 1999-2000 to 2010-2011 seasons. Available at: http://www.cdc.gov/flu/weekly/pastreports.htm. Accessed November 17, 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
