Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy
- PMID: 22252008
- PMCID: PMC3336076
- DOI: 10.4161/auto.8.2.18554
Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy
Abstract
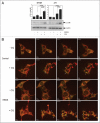
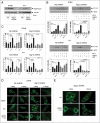
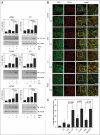
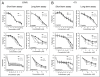
Chloroquine (CQ) is a 4-aminoquinoline drug used for the treatment of diverse diseases. It inhibits lysosomal acidification and therefore prevents autophagy by blocking autophagosome fusion and degradation. In cancer treatment, CQ is often used in combination with chemotherapeutic drugs and radiation because it has been shown to enhance the efficacy of tumor cell killing. Since CQ and its derivatives are the only inhibitors of autophagy that are available for use in the clinic, multiple ongoing clinical trials are currently using CQ or hydroxychloroquine (HCQ) for this purpose, either alone, or in combination with other anticancer drugs. Here we show that in the mouse breast cancer cell lines, 67NR and 4T1, autophagy is induced by the DNA damaging agent cisplatin or by drugs that selectively target autophagy regulation, the PtdIns3K inhibitor LY294002, and the mTOR inhibitor rapamycin. In combination with these drugs, CQ sensitized to these treatments, though this effect was more evident with LY294002 and rapamycin treatment. Surprisingly, however, in these experiments CQ sensitization occurred independent of autophagy inhibition, since sensitization was not mimicked by Atg12, Beclin 1 knockdown or bafilomycin treatment, and occurred even in the absence of Atg12. We therefore propose that although CQ might be helpful in combination with cancer therapeutic drugs, its sensitizing effects can occur independently of autophagy inhibition. Consequently, this possibility should be considered in the ongoing clinical trials where CQ or HCQ are used in the treatment of cancer, and caution is warranted when CQ treatment is used in cytotoxic assays in autophagy research.
Figures



Similar articles
-
Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion.Autophagy. 2018;14(8):1435-1455. doi: 10.1080/15548627.2018.1474314. Epub 2018 Jul 20. Autophagy. 2018. PMID: 29940786 Free PMC article.
-
Abrogation of Autophagy by Chloroquine Alone or in Combination with mTOR Inhibitors Induces Apoptosis in Neuroendocrine Tumor Cells.Neuroendocrinology. 2016;103(6):724-37. doi: 10.1159/000442589. Epub 2015 Dec 1. Neuroendocrinology. 2016. PMID: 26619207
-
Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy.Neurosurg Focus. 2014 Dec;37(6):E12. doi: 10.3171/2014.9.FOCUS14504. Neurosurg Focus. 2014. PMID: 25434381
-
Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms.Pharmacol Res. 2021 Jun;168:105582. doi: 10.1016/j.phrs.2021.105582. Epub 2021 Mar 26. Pharmacol Res. 2021. PMID: 33775862 Review.
-
Repurposing Chloroquine Analogs as an Adjuvant Cancer Therapy.Recent Pat Anticancer Drug Discov. 2021;16(2):204-221. doi: 10.2174/1574892815666210106111012. Recent Pat Anticancer Drug Discov. 2021. PMID: 33413069 Review.
Cited by
-
Autophagy-related gene 12 (ATG12) is a novel determinant of primary resistance to HER2-targeted therapies: utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment.Oncotarget. 2012 Dec;3(12):1600-14. doi: 10.18632/oncotarget.742. Oncotarget. 2012. PMID: 23307622 Free PMC article.
-
Polymeric chloroquine as an inhibitor of cancer cell migration and experimental lung metastasis.J Control Release. 2016 Dec 28;244(Pt B):347-356. doi: 10.1016/j.jconrel.2016.07.040. Epub 2016 Jul 27. J Control Release. 2016. PMID: 27473763 Free PMC article.
-
Alternative Splicing and Cleavage of GLUT8.Mol Cell Biol. 2020 Dec 21;41(1):e00480-20. doi: 10.1128/MCB.00480-20. Print 2020 Dec 21. Mol Cell Biol. 2020. PMID: 33077497 Free PMC article.
-
PTRF/Cavin-1 enhances chemo-resistance and promotes temozolomide efflux through extracellular vesicles in glioblastoma.Theranostics. 2022 May 16;12(9):4330-4347. doi: 10.7150/thno.71763. eCollection 2022. Theranostics. 2022. PMID: 35673568 Free PMC article.
-
Yeast β-D-glucan exerts antitumour activity in liver cancer through impairing autophagy and lysosomal function, promoting reactive oxygen species production and apoptosis.Redox Biol. 2020 May;32:101495. doi: 10.1016/j.redox.2020.101495. Epub 2020 Mar 7. Redox Biol. 2020. PMID: 32171725 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
