Robust, small-scale cultivation platform for Streptomyces coelicolor
- PMID: 22252012
- PMCID: PMC3292921
- DOI: 10.1186/1475-2859-11-9
Robust, small-scale cultivation platform for Streptomyces coelicolor
Abstract
Background: For fermentation process and strain improvement, where one wants to screen a large number of conditions and strains, robust and scalable high-throughput cultivation systems are crucial. Often, the time lag between bench-scale cultivations to production largely depends on approximate estimation of scalable physiological traits. Microtiter plate (MTP) based screening platforms have lately become an attractive alternative to shake flasks mainly because of the ease of automation. However, there are very few reports on applications for filamentous organisms; as well as efforts towards systematic validation of physiological behavior compared to larger scale are sparse. Moreover, available small-scale screening approaches are typically constrained by evaluating only an end point snapshot of phenotypes.
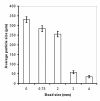
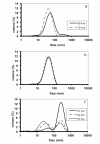
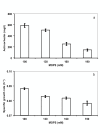
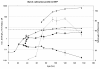
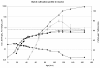
Results: To address these issues, we devised a robust, small-scale cultivation platform in the form of MTPs (24-square deepwell) for the filamentous bacterium Streptomyces coelicolor and compared its performance to that of shake flasks and bench-scale reactors. We observed that re-designing of medium and inoculum preparation recipes resulted in improved reproducibility. Process turnaround time was significantly reduced due to the reduction in number of unit operations from inoculum to cultivation. The incorporation of glass beads (ø 3 mm) in MTPs not only improved the process performance in terms of improved oxygen transfer improving secondary metabolite production, but also helped to transform morphology from pellet to disperse, resulting in enhanced reproducibility. Addition of MOPS into the medium resulted in pH maintenance above 6.50, a crucial parameter towards reproducibility. Moreover, the entire trajectory of the process was analyzed for compatibility with bench-scale reactors. The MTP cultivations were found to behave similar to bench-scale in terms of growth rate, productivity and substrate uptake rate and so was the onset of antibiotic synthesis. Shake flask cultivations however, showed discrepancy with respect to morphology and had considerably reduced volumetric production rates of antibiotics.
Conclusion: We observed good agreement of the physiological data obtained in the developed MTP platform with bench-scale. Hence, the described MTP-based screening platform has a high potential for investigation of secondary metabolite biosynthesis in Streptomycetes and other filamentous bacteria and the use may significantly reduce the workload and costs.
Figures
Similar articles
-
Reducing the variability of antibiotic production in Streptomyces by cultivation in 24-square deepwell plates.J Biosci Bioeng. 2010 Mar;109(3):230-4. doi: 10.1016/j.jbiosc.2009.08.479. Epub 2009 Sep 16. J Biosci Bioeng. 2010. PMID: 20159569
-
Fast and reliable strain characterization of Streptomyces lividans through micro-scale cultivation.Biotechnol Bioeng. 2017 Sep;114(9):2011-2022. doi: 10.1002/bit.26321. Epub 2017 May 23. Biotechnol Bioeng. 2017. PMID: 28436005
-
Process performance of parallel bioreactors for batch cultivation of Streptomyces tendae.Bioprocess Biosyst Eng. 2011 Mar;34(3):297-304. doi: 10.1007/s00449-010-0471-1. Epub 2010 Oct 8. Bioprocess Biosyst Eng. 2011. PMID: 20931236
-
Early phase process scale-up challenges for fungal and filamentous bacterial cultures.Appl Biochem Biotechnol. 2004 Dec;119(3):241-78. doi: 10.1007/s12010-004-0005-x. Appl Biochem Biotechnol. 2004. PMID: 15591617 Review.
-
Structured morphological modeling as a framework for rational strain design of Streptomyces species.Antonie Van Leeuwenhoek. 2012 Oct;102(3):409-23. doi: 10.1007/s10482-012-9760-9. Epub 2012 Jun 21. Antonie Van Leeuwenhoek. 2012. PMID: 22718122 Free PMC article. Review.
Cited by
-
Synthetic promoter library for modulation of actinorhodin production in Streptomyces coelicolor A3(2).PLoS One. 2014 Jun 25;9(6):e99701. doi: 10.1371/journal.pone.0099701. eCollection 2014. PLoS One. 2014. PMID: 24963940 Free PMC article.
-
Improved Time Resolved KPI and Strain Characterization of Multiple Hosts in Shake Flasks Using Advanced Online Analytics and Data Science.Bioengineering (Basel). 2022 Jul 25;9(8):339. doi: 10.3390/bioengineering9080339. Bioengineering (Basel). 2022. PMID: 35892752 Free PMC article.
-
Quantification and modeling of macroparticle-induced mechanical stress for varying shake flask cultivation conditions.Front Bioeng Biotechnol. 2023 Sep 4;11:1254136. doi: 10.3389/fbioe.2023.1254136. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37731767 Free PMC article.
-
Microtiter plate-based cultivation to investigate the growth of filamentous fungi.Eng Life Sci. 2017 Jul 20;17(10):1064-1070. doi: 10.1002/elsc.201700041. eCollection 2017 Oct. Eng Life Sci. 2017. PMID: 32624733 Free PMC article.
-
Development of an agar-plug cultivation system for bioactivity assays of actinomycete strain collections.PLoS One. 2021 Nov 5;16(11):e0258934. doi: 10.1371/journal.pone.0258934. eCollection 2021. PLoS One. 2021. PMID: 34739482 Free PMC article.
References
-
- Kisser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. Norwich, England: The John Innes Foundation; 2000.
-
- Baltz RH. Genetic methods and strategies for secondary metabolite yield improvement in actinomycetes. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology. 2001;79(3-4):251–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

