A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death
- PMID: 22252128
- PMCID: PMC3298003
- DOI: 10.1038/emboj.2011.497
A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death
Abstract
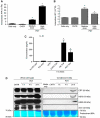
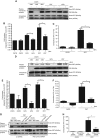
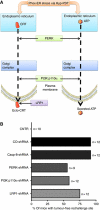
Surface-exposed calreticulin (ecto-CRT) and secreted ATP are crucial damage-associated molecular patterns (DAMPs) for immunogenic apoptosis. Inducers of immunogenic apoptosis rely on an endoplasmic reticulum (ER)-based (reactive oxygen species (ROS)-regulated) pathway for ecto-CRT induction, but the ATP secretion pathway is unknown. We found that after photodynamic therapy (PDT), which generates ROS-mediated ER stress, dying cancer cells undergo immunogenic apoptosis characterized by phenotypic maturation (CD80(high), CD83(high), CD86(high), MHC-II(high)) and functional stimulation (NO(high), IL-10(absent), IL-1β(high)) of dendritic cells as well as induction of a protective antitumour immune response. Intriguingly, early after PDT the cancer cells displayed ecto-CRT and secreted ATP before exhibiting biochemical signatures of apoptosis, through overlapping PERK-orchestrated pathways that require a functional secretory pathway and phosphoinositide 3-kinase (PI3K)-mediated plasma membrane/extracellular trafficking. Interestingly, eIF2α phosphorylation and caspase-8 signalling are dispensable for this ecto-CRT exposure. We also identified LRP1/CD91 as the surface docking site for ecto-CRT and found that depletion of PERK, PI3K p110α and LRP1 but not caspase-8 reduced the immunogenicity of the cancer cells. These results unravel a novel PERK-dependent subroutine for the early and simultaneous emission of two critical DAMPs following ROS-mediated ER stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Comment in
-
Enlightening the impact of immunogenic cell death in photodynamic cancer therapy.EMBO J. 2012 Mar 7;31(5):1055-7. doi: 10.1038/emboj.2012.2. Epub 2012 Jan 17. EMBO J. 2012. PMID: 22252132 Free PMC article.
Similar articles
-
Biphasic Increases of Cell Surface Calreticulin Following Treatment with Mitoxantrone.Biol Pharm Bull. 2020 Oct 1;43(10):1595-1599. doi: 10.1248/bpb.b20-00319. Epub 2020 Jul 29. Biol Pharm Bull. 2020. PMID: 32727970
-
Immunogenic cell death and DAMPs in cancer therapy.Nat Rev Cancer. 2012 Dec;12(12):860-75. doi: 10.1038/nrc3380. Epub 2012 Nov 15. Nat Rev Cancer. 2012. PMID: 23151605 Review.
-
Wogonin induced calreticulin/annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner.PLoS One. 2012;7(12):e50811. doi: 10.1371/journal.pone.0050811. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251389 Free PMC article.
-
ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death.Autophagy. 2013 Sep;9(9):1292-307. doi: 10.4161/auto.25399. Epub 2013 Jun 19. Autophagy. 2013. PMID: 23800749
-
The PERKs of damage-associated molecular patterns mediating cancer immunogenicity: From sensor to the plasma membrane and beyond.Semin Cancer Biol. 2015 Aug;33:74-85. doi: 10.1016/j.semcancer.2015.03.010. Epub 2015 Apr 13. Semin Cancer Biol. 2015. PMID: 25882379 Review.
Cited by
-
Low dose of GRP78-targeting subtilase cytotoxin improves the efficacy of photodynamic therapy in vivo.Oncol Rep. 2016 Jun;35(6):3151-8. doi: 10.3892/or.2016.4723. Epub 2016 Apr 1. Oncol Rep. 2016. PMID: 27035643 Free PMC article.
-
Editorial: Immunogenic Cell Death in Cancer: From Benchside Research to Bedside Reality.Front Immunol. 2016 Mar 29;7:110. doi: 10.3389/fimmu.2016.00110. eCollection 2016. Front Immunol. 2016. PMID: 27066003 Free PMC article. No abstract available.
-
Many faces of DAMPs in cancer therapy.Cell Death Dis. 2013 May 16;4(5):e631. doi: 10.1038/cddis.2013.156. Cell Death Dis. 2013. PMID: 23681226 Free PMC article. Review.
-
Trial watch: Chemotherapy with immunogenic cell death inducers.Oncoimmunology. 2013 Mar 1;2(3):e23510. doi: 10.4161/onci.23510. Oncoimmunology. 2013. PMID: 23687621 Free PMC article.
-
Purinergic signalling and cancer.Purinergic Signal. 2013 Dec;9(4):491-540. doi: 10.1007/s11302-013-9372-5. Purinergic Signal. 2013. PMID: 23797685 Free PMC article. Review.
References
-
- Agostinis P, Vantieghem A, Merlevede W, de Witte PA (2002) Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol 34: 221–241 - PubMed
-
- Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L (2010) Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 70: 855–858 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

